Snail research – optimising control
Author: Helen Brodie, Greg Baker, Kate Muirhead and Kym Perry (Entomology Unit, South Australian Research and Development Institute) | Date: 14 Feb 2019
Take home messages
- Effective snail baiting requires applying baits at the right time and at sufficient pellet density to ensure good encounter rates.
- Bait in autumn as soon as snails become active and before they lay eggs, check spreader calibration, apply an adequate rate and select an appropriate product for the field conditions.
Background
Four introduced snail species of European-Mediterranean origin have established in southern Australia and become major pests of grain crops. These snails are: vineyard or common white snail [Cernuella virgata (Da Costa) (Hygromiidae)], pointed snail [Cochlicella acuta (Müller) (Hygromiidae)], small pointed snail [Prietocella barbara (L.) (Hygromiidae)], and the white Italian snail [Theba pisana (Müller) (Helicidae)]. The market access threat from these snails is substantial and increasing, particularly for the acceptance of Australian wheat and barley shipments by valuable east Asian markets, e.g. China, South Korea. The shift to minimal soil cultivation, retained stubbles and limited grazing has advantaged snail survival and reproduction in this system, and many of the harvester modifications and summer cultural controls developed and extended in the early 2000s (Bash ‘Em, Burn ‘Em, Bait ‘Em;) have become increasingly incompatible with current farming practice.
Recent SARDI research in GRDC projects DAS00134 and YPA00002 focused on improving baiting performance by investigating the factors influencing the performance of commercial molluscicidal baits against different densities of the four snail species and under different environmental conditions. The current GRDC project DAS00160 is investigating the environmental factors influencing snail movement, feeding and reproductive activity to assist growers optimise the timing of baiting programs. The work has revealed unexpected findings suggesting that the susceptibility of snails to baits (and hence the efficiency of baiting) can change through the season. The past five years of research has generated refined baiting guidelines for snails and slugs which will be available in updated publications (e.g. Bash’em, Burn’em, Bait’em) by the end of 2019. In addition, a current GRDC- CSIRO-SARDI project (CSE00061) is aiming to improve biological control of the conical snail by potentially introducing a new strain of the parasitoid fly, Sarcophaga villeneuveana.
This paper summarises research findings on recent projects and preliminary observations on current projects.
Methods
Under project DAS00160 (2017-June 2019), microclimate effects on snail movement and reproductive activity are being measured at six field sites across South Australia and Western Australia). In addition to using micro-climate sensors and fixed cameras to monitor snail movement, common white snails and small-pointed snails (approx. 150) have been collected monthly at each site and dissected to determine their reproductive stage by measuring their albumen glands. Swollen albumen glands indicate snails are reproductively active. Changes in the susceptibility of snails to bait during the season have been measured in laboratory trials. This report presents preliminary data of common white snails collected from Palmer and Urania SA. Snails were placed into moist arenas (500mL ventilated plastic food containers with moist substrate, five snails per arena, 50 snails per treatment) in a laboratory environment (21°C) and provided with either pre-weighed Metarex® or placebo baits (Metarex® minus the active ingredient). Baits were removed after 2-3 nights and mortality assessed after a further 4-5 nights. Bait consumption and corresponding snail weights, body moisture, shell size and reproductive stage were determined (not presented).
Results and discussion
Snail movement and reproductive activity
In autumn, an increase in rainfall and dew events stimulates increasing snail activity. Dissection of monthly-collected common white snails in SA over the past three seasons has shown that the reproductive organs (albumen glands) of common white snails begin to enlarge from late March onwards, and then most reproductive activity occurs from late April to July (Figure 1). Depending on how quickly the season dries up, an increasing proportion of snails cease reproduction late winter and early spring and remain inactive until the following season. For growers, this suggests that under typically dry conditions, common white snail movement events prior to mid-March are unlikely to involve significant reproductive activity (noting that further testing under wetter summer conditions is required). Similar gland size patterns also occur in white Italian snails at Warooka, SA (preliminary gland monitoring December 2014-May 2017, data not presented here).
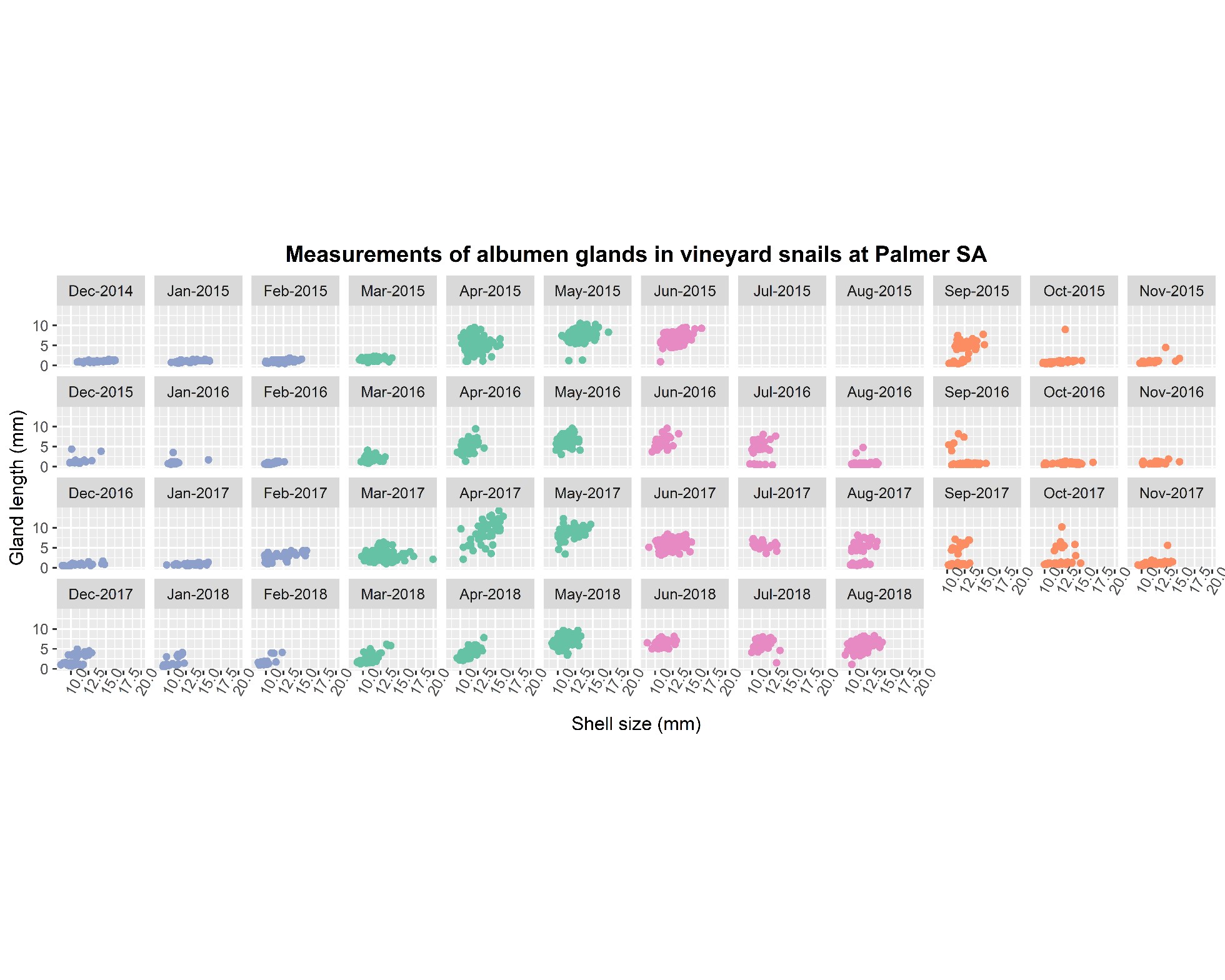
Figure 1. Albumen gland length (mm) and snail shell diameter (mm) plotted for each month for common white snails at Palmer, SA.
Controlling snails with molluscicides
Snails become inactive when they experience unfavourable (i.e. dry, low relative humidity) micro-climatic conditions. This ‘on-off’ behaviour makes them a difficult target for chemical control. The international literature indicates that molluscicide baits are generally more effective than sprays, and this appears to be because baits have greater persistence and hence greater likelihood of being encountered by the snails when they do become active.
Laboratory baiting experiments on mature common white snails suggest that snails may respond differently to the ingestion of Metarex® baits (5% metaldehyde) depending on the time of year. Mortality varied between approx. 30% to 75% for snails from Palmer and approx. 10% to 80% for snails from Urania (Figure 2). Higher kills were observed amongst snails collected during autumn and winter than in spring or summer, despite similar levels of bait consumption. Variation in the amount of Metarex® consumed (not presented) did not display any seasonal pattern nor correlate with mortality.
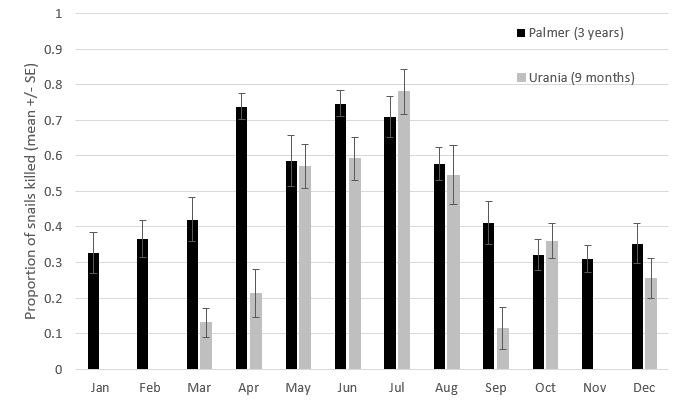
Figure 2. Mean mortality (± standard error) of mature common white snails collected monthly and offered Metarex® bait in consistent laboratory conditions. Palmer, SA data is monthly averages from March 2016 through to December 2018. Urania, SA data is monthly from March 2018 to December 2018. Data corrected for control mortality. For each sampled date, n = 50 and control mortality 0-6%.
The Palmer 3-year data set shows that bait efficacy in killing snails increases sharply between March and April, corresponding with commencement of high snail activity (including mating and egg-laying). This distinct activity peak in April was not replicated in the Urania 9-month data set, which may be a result of the relatively small data set and limited rainfall (19.8mm) in April at Maitland, SA (BOM station 22088) that occurred after the snails had been sampled (i.e. after the 12th April). The data suggest snails may be metabolically more susceptible to metaldehyde during periods of reproductive activity. For growers, the implication is that baiting may be most efficient from April to approx. July, and less efficient at other times. However, it is more important to prevent breeding (juveniles are extremely hard to control with baits) than to restrict baiting to the April-July period since some breeding may happen earlier if regular rain/dew events occur. The reduced susceptibility to metaldehyde in spring and summer is a good reason to avoid ‘last-ditch-effort’ pre-harvest baiting. Late season baiting also increases the risk of bait contaminating grain at harvest.
Optimising bait operations
Below are general guidelines that have emerged from the recent body of snail research.
Bait encounter is random. Therefore, the effectiveness of a snail baiting program is governed by a number of factors that firstly dictate chance of encounter, then ingestion of the toxicant:
- Chance of encounter
- Level of snail activity
- Attractiveness of bait
- Baits per unit area.
- Ingestion of lethal dose
- Palatability of bait
- Quantity of bait
- Adequate active ingredient.
Hence, it is recommended that baiting programs take place when snails are active and that enough bait ‘points’ are provided to ensure good rates of encounters. The number of baits on the ground is of equal, if not greater importance, than weight of product on the ground.
Apply baits at the right time
- Baiting must occur in autumn as soon as snails become active, but before they lay eggs. Research results strongly support concentrating baiting efforts in autumn for several reasons: Preventing adult snails from laying eggs is critical to reduce population build-up. Juvenile snails are generally more difficult to control using baits due to reduced movement and bait encounter.
- Laboratory trials at SARDI have found higher efficacy when baits were applied under warmer temperatures within the range tested (10oC-22oC).
- Baiting is most efficient when there is less ground cover and alternative food. The presence of stubble, weeds and crop plants at later times reduces bait encounter.
- The susceptibility of snails to metaldehyde baits appears to be highest between April and July (Figure 2).
In autumn, even light showers or overnight dews are sufficient to stimulate movement. Ideal conditions for baiting are periods when the soil is likely to remain moist for several days. If unsure whether snails are active, bait a small area and check for dead snails after a few days. Even if snails do not look active during the day, slime trails across the soil surface can be a good indicator of night activity.
Apply an adequate rate of bait
Based on SARDI research, regardless of product used a minimum of 30 bait pellets per square metre and up to 60 pellets per square metre at very high snail densities, should be applied to ensure a sufficient density of bait points and chance of encounter. The higher rates may be needed in heavily infested areas, such as perimeters, fence lines or calcareous outcrops. Where current label rates do not permit this, a repeat application should be considered. Pellet densities for registered rates of commercial products are available in the SARDI Snail and Slug baiting guidelines brochure (refer to the Useful Resources section of this paper). Monitor live snail densities and re-apply bait as necessary. A repeat application may be needed in areas with high snail densities or when rainfall has broken down bait.
Spreader calibration important to achieve even bait distribution and good results
Do not assume your spreader is distributing bait pellets evenly. Research by the Yorke Peninsula Alkaline Soils Group and SARDI has shown spreaders calibrated for other applications (e.g. urea) may not broadcast baits as widely as expected, and ute spreaders may provide uneven distribution of bait. Different bait products also have different hardness and ballistic properties.
Therefore:
- For your preferred bait product, have your spreader professionally calibrated to evenly broadcast the target pellet density over the entire spread width.
- Operators should actively check pellet distribution across the entire spread width.
- The single spinner ute spreaders generally perform poorly with limited spread widths and uneven bait distribution.
Be aware of potential bait degradation
Some bait products are more stable under adverse weather conditions, such as cold temperatures and rainfall. Significant rainfall can degrade bran-based pellets and reduce efficacy, particularly for iron chelate products. SARDI trials found that UV exposure did not reduce the efficacy of baits in summer and early autumn, however, exposure to high temperatures (above 30oC–40oC) degraded the active ingredient in metaldehyde baits. Avoid storing bait for long periods in places where temperatures exceed 35oC (e.g. hot sheds).
Progress on biological control of conical snail
The parasitic fly, Sarcophaga villeneuveana, was released in SA during 2001-2004 at 21 sites (19 on Yorke Peninsula and two sites on the Limestone Coast) to control conical snails (Cochlicella acuta) (Hopkins 2005; Leyson et al.2003; Coupland & Baker 2007). The flies established on Southern Yorke Peninsula, but to this day have displayed low parasitism rates and limited natural dispersal, with little impact on pest snail populations. CSIRO work has since determined that the fly population previously released in Australia parasitises a different host strain in its native (southern France) habitat, which may explain its poor performance in Australia. In a new GRDC project, CSIRO and SARDI aim to enhance the biological control provided by S. villeneuveana by introducing a different strain of the fly from European locations where the correct lineage of C. acuta predominates and the climate is more similar to SA.
Conclusion
Current research is strongly supporting concentrating baiting programs in autumn and early winter, due to (1) the need to prevent breeding, (2) to maximise the efficiency of baiting, by baiting when there is minimal alternative food, and when snail mortality response to baits appears to be highest.
Snail movement (i.e. baiting opportunities) occurs anytime there is adequate moisture, but under average seasonal conditions, minimal reproduction appears to occur prior to mid-March (note: yet to be assessed under very wet summers). Growers can aim to focus baiting efforts in the March to April period to pre-empt snail breeding. Baiting after late winter is likely to be less efficient and should cease at least two months prior to harvest to avoid bait contamination of grain (zero tolerance).
New baiting guidelines, incorporating new information on movement triggers from the camera/sensor work, will be made available to growers by the end of 2019 in a new version of the ‘Bash ‘em Burn ‘em Bait ‘em manual.
Baiting during autumn is a key component of a year-round systems approach, which is critical for effective snail management. Baiting should be used in conjunction with cultural controls during summer and autumn to reduce snail survival, such as cabling, rolling and/or burning, or summer grazing, along with effective weed control to remove refuge habitat.
The key to successful snail control is year-round integrated management:
- Continuous vigilance.
- Prepare in advance.
- Remove summer refuges.
- Roll or cable in summer when > 35oC.
- Bait before egg laying occurs.
- Baiting in winter and spring is less effective.
- Harvester modifications.
- Grain cleaning last resort.
Useful resources
http://www.pir.sa.gov.au/__data/assets/pdf_file/0004/286735/Snail_and_slug_baiting_guidelines.pdf
https://grdc.com.au/__data/assets/pdf_file/0024/117249/grdc-fs-snailbait-south_lr-pdf.pdf.pdf
https://grdc.com.au/__data/assets/pdf_file/0016/109060/snail-management-fact-sheet.pdf.pdf
References
Coupland JB & Baker GH. 2007. Search for biological control agents of invasive Mediterranean snails. In: Vincent C, Goettel MS, Lazarovits G. (eds). Biological Control: A Global Perspective. CAB International, Wallingford, UK. Pp.7-12.
Hopkins DC. 2005. Final report for the Grains Research and Development Corporation Project DAS300 ’Integrated Snail Management in the Southern Region’
Leyson M, Hopkins DC & Charwat S. 2003. Release and establishment in South Australia of Sarcophaga pencillata (Diptera: Sarcophagidae), a biological control agent for Cochlicella acuta (Mollusca: Hygromiidae). BCPC Symposium Proceedings No. 80: Slugs & Snails: Agricultural, Veterinary & Environmental Perspectives. Pp 295-300.
Acknowledgements
The research undertaken as part of this project (DAS00134 and DAS00160) and others referred to in this paper (DAS300 and YPA0002) is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC — the author would like to thank them for their continued support.
Contact details
Helen Brodie
SARDI Entomology, Waite Campus, Adelaide
08 8429 0557
helen.brodie@sa.gov.au
Was this page helpful?
YOUR FEEDBACK
