Residue Watch: How do I know if herbicide residues are breaking down before sowing
Residue Watch: How do I know if herbicide residues are breaking down before sowing
Take home messages
- Herbicide residues can be measured in soil, but information about crop toxicity thresholds and soil-specific herbicide availability is also needed to interpret what soil analysis results mean for the crop. Some of this data is now available for imidazolinone herbicides.
- Sorption of imidazolinone herbicides is low in alkaline soils, even in alkaline clay soil types.
- Soil analysis can provide an extra layer of information for decision making, but there will always be uncertainty in interpreting potential crop effects due to in-paddock variation, environmental conditions, and cultivar-specific tolerances.
- Soil analysis for herbicide residues is not a replacement for herbicide label directions.
Background
Herbicides with soil residual activity are being used extensively to help counter the evolution and spread of herbicide-resistant weed populations. Imidazolinone herbicides (‘imis’) such as imazapyr and imazamox are used in the southern and western grain regions for weed control in combination with imi-tolerant crops. Although residual herbicides provide a longer control period for certain weeds, their persistence in the soil for lengthy periods (12 – 24 months) may impede the growth of subsequent winter crops (e.g. barley, wheat, chickpea, lentils, field peas, lupins) (Fleming et. al. 2012). Because of concerns around herbicide carryover affecting subsequent crops, there can be a loss in flexibility of crop rotations. There are also reports of crop damage attributed to residual herbicide carryover outside the label plant-back guidelines.
The difficulty in providing a more accurate guide for determining when herbicide residues will cause crop damage is related to several factors:
- There is little or no accurate information available on crop toxicity thresholds for many herbicides, in terms of a measured concentration of herbicide in soil (e.g. mg herbicide per kg of soil)
- The bioavailable levels of herbicides (i.e how much the plant roots actually access) will vary from soil to soil, depending on herbicide and soil chemistry. Current soil tests nearly always report total, rather than available, herbicide residue concentrations.
- Other environmental factors can influence the herbicide toxicity, including weather conditions (e.g. frost), soil properties (e.g. micronutrient availability) and plant-specific characteristics (e.g. cultivar tolerance).
As a result, farmers do not have the information or tools available to determine residue levels in their soils and to make informed decisions relating to the planting of subsequent crops – aside from relying on product labels or conducting their own bioassays. Crop toxicity thresholds combined with accurate and timely detection or prediction of residue levels could provide farmers and consultants with an added layer of information to make more confident decisions on the use of residual herbicides in their farming systems. In many cases this may just be in the form of confirming observations or ‘gut-feel’ about herbicide behaviour with chemical test results, helping to reduce uncertainty about the cause for poor crop performance. This paper reports on findings from two concurrent projects (GRDC and Soil CRC) aiming to i) define crop toxicity thresholds and ii) work toward developing a potential test for residue levels of imidazolinone and other residual herbicides.
Method
Approach
Commercially available herbicide testing methods provide a measure of the total herbicide concentration in soil - but depending on the soil type, only a fraction of the total herbicide concentration is bioavailable to the crop. This means that in one soil type, a total residue concentration of 10ng/g may be toxic, whilst in another soil type, 10ng/g may be non-toxic. A more relevant measure is the bioavailable herbicide concentration. However, there are very few validated methods for determining the bioavailable herbicide concentration in soil.
One way to estimate the bioavailable fraction is to determine the sorption coefficient (known as the Kd) for each soil type, which is defined as the proportion of bound herbicide (unavailable) versus the proportion of soluble herbicide (bioavailable). However, determining the Kd for a specific soil type is quite tedious and costly. Rather than determining the Kd for every soil type via a laboratory experiment, this project aims to use soil properties and/or mid-infrared reflectance (MIR) spectroscopy (a rapid and relatively inexpensive test) to estimate the Kd based on a calibration set of soils. Estimates of bioavailable herbicide residues can then be compared to dose-response toxicity curves for different crops that have been calibrated for either i: maximum bioavailability (i.e. crops grown in sand) thresholds, or ii: soil-specific bioavailability thresholds. The approach is summarised in Figure 1.
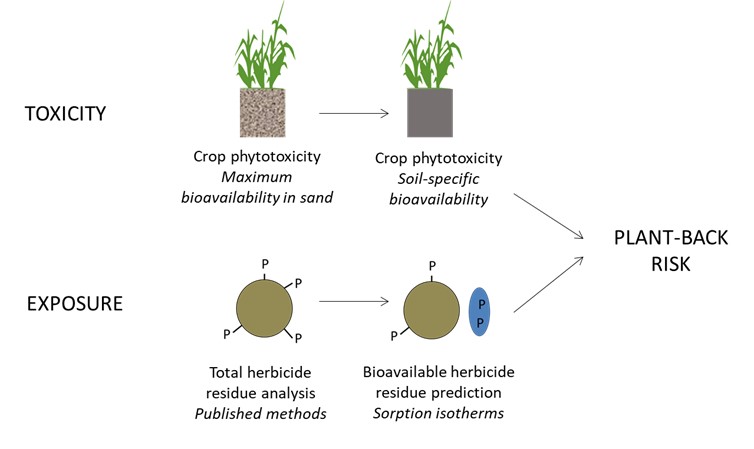
Figure 1. Framework to improve prediction of plant-back risk.
Seedling toxicity thresholds to imazapyr and imazapic
Washed pure sand and topsoil samples taken in 2019 prior to herbicide application were transported to NSW DPI Wollongbar, air dried, homogenised and sieved to < 2mm. Subsamples of sand or soil (20kg) were then spiked with increasing concentrations of herbicide, with six levels ranging from 0 – 30ng/g for imazapyr, and then aged for 1 week at room temperature. Residue levels were confirmed by liquid chromatography/mass spectrometry. Wheat (Scepter), barley (La Trobe), canola (Diamond), lupin (PBA Batemen), field pea (PBA Butler), chickpea (PBA Slasher), faba bean (Nasma) and lentil (PBA Bolt) were sown into pots (dimensions 65mm by 65mm and 160mm depth, filled with 140mm sand/soil kept moist to 80% field capacity) and harvested after 21 days after sowing. Shoots were cut at the soil surface, weighed, dried at 60°C for two days and then re-weighed to determine dry weight. Dose-response thresholds were determined by fitting shoot dry weight data to soil clopyralid concentrations using four parameter log-logistic curves and the ‘effective dose’ for 20% shoot biomass reduction (ED20) was calculated.
Sorption of imazapyr and imazapic to soils
Soils were spiked with three levels of each herbicide representing concentrations that would be present in the soil profile immediately after a label rate application (i.e. in the top 1 cm) and subsequent dissipation. This was equivalent 10, 50 and 250ng/g for imazapic and imazapyr. Sorption isotherms were measured as per OECD guidelines (OECD 2000) after optimising sorption kinetics (time to reach equilibrium, 18 hours) and soil: solution concentrations (1:2 soil: solution). After centrifuging, the concentration of herbicide remaining in the soil solution was determined by LC-MS/MS and the amount of adsorbed herbicide calculated by difference. Plots of adsorbed concentration vs dissolved concentration were fit with linear adsorption isotherms to calculate sorption coefficients. Soil physicochemical characteristics (pHCaCl2; organic carbon and cation exchange capacity) were used to fit linear regression models for predicting soil sorption coefficients.
Case study analysis of field soils
Pre-sowing samples from Southern region sites
Participating growers were invited to submit topsoil (0-10 cm) samples taken from paddocks with a history of imi-herbicide application where there were concerns of potential carryover. Four soil samples from each paddock were taken from a 50 x 50 m grid in April 2021, where each sample was a composite of three homogenised subsamples. Samples were transported to NSW DPI Wollongbar and analysed for imi-herbicides using a modified QuECHERs extraction and LC-MS/MS quantification. In addition, spike-recoveries were conducted for each soil sample for quality control purposes.
Results and discussion
Dose-response thresholds
Imazapyr showed significantly greater toxicity to wheat, barley and canola in comparison to all legumes as shown by lower toxicity thresholds in table 1. Toxicity thresholds were lower in sand than in the two field soils, but the extent differed between species. For example, the toxicity of imazapyr residues to wheat was higher than barley and canola in pure sand, but the reverse was true in soil. Thresholds were also higher in the soil with more clay and lower pH (i.e. the Birchip soil) than the soil with lower clay content and higher pH (i.e. the Minnipa soil). This trend fits the prediction of greater sorption of imazapyr in the Birchip soil.
Table 1. Imazapyr toxicity thresholds (ng/g) for 20% shoot dry weight reductions (ED20).
Species | Sand (0% clay) | Minnipa Soil (6% clay, pH 7.8, OC = 1%) | Birchip Soil (14% clay, pH 7.4, OC = 1.1%) |
|---|---|---|---|
Lentil | >30 | >30 | >30 |
Field pea | 21 | >30 | >30 |
Lupin | 19 | >30 | >30 |
Chickpea | >30 | >30 | >30 |
Faba bean | 3.7 | N/A | N/A |
Wheat | 1 | 1.3 | 4.2 |
Barley | 0.2 | 1.7 | 5.5 |
Canola | 0.1 | 3.9 | 6.2 |
To date, toxicity thresholds for imazapic have only been conducted in pure sand. Toxicity thresholds here have been based on a rapid root elongation method and are not directly comparable with imazapyr thresholds. Note that ED30, rather than ED20, values are shown (table 2). Of note is the sensitivity of sorghum to imazapic residues, which is more than twice as sensitive compared with other crop species tested.
Table 2. Imazapic toxicity thresholds (ng/g) for 30% root length reductions (ED30) for crops grown in sand. *Average of two individual dose-response experiments.
Species | Sand (0% clay) |
|---|---|
Chickpea | 21 |
Mungbean | 28 |
Wheat | 20 |
Barley | 11 |
Maize | 13* |
Sorghum | 3.7 |
Measurement and prediction of soil sorption coefficients (Kd) in different soil
Imazapyr sorption coefficients were determined for 48 soils taken from the Victorian Mallee (Vic), Eyre Peninsula (SA) and Western Australian wheatbelt. Sorption coefficients were low (<1L kg-1 for all soils tested), confirming that imazapyr is mobile in these Australian cropping soils. The Kd values determined here are within the range of those determined in other studies: 0.07 - 0.19L/kg for 5 Alabama soils (Wehtje et al. 1987) and <0.1-2.8L/kg for a number of Argentinean soils (Gianelli et al. 2014; Porfiri et al. 2015).
The soil sorption coefficients determined here were negatively correlated to soil pH (Figure 2A), that is, sorption was generally greater in acid soils. This is similar to previous research demonstrating significant negative correlations of imazapic sorption with pH and positive correlations with organic carbon (OC) and clay content. It is likely that the limited variation in physicochemical properties (i.e. predominantly low OC, neutral-alkaline soils) and the low Kd values precluded us from other potential relationships with clay or OC.
In contrast, the measured sorption partition coefficients (Kd) for imazapic on 42 soils from the northern grains region were generally higher than for imazapyr, ranging from 0.1 – 5.4L/kg. The majority of soil (90%) had a Kd of less than 2L/kg. Interestingly, there are very few published Kd values for imazapic – for example, there is no data for imazapic soil adsorption (linear or Freundlich) in the pesticides properties database (Pesticide Properties DataBase). Linear regression with multiple soil properties found that pH, OC, cation exchange capacity and their interactions improved predictions of imazapyr and imazapic sorption (Figure 2) compared to single correlations. As with imazapyr, sorption was generally greater in acid soils, especially in acid soils with higher organic carbon and clay content. These soil properties are measured as part of most soil tests, which means that the sorption coefficient (Kd) for imazapic can be estimated for northern region soil types where this data already exists.
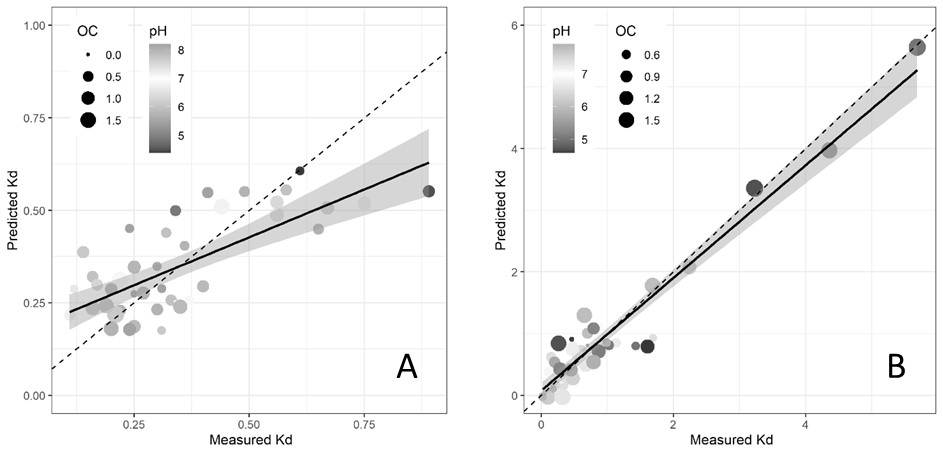
Figure 2. Measured and predicted sorption of (A) imazapyr to southern region soils (n=48) and (B) imazapic to northern region soils (n=42). Note the different scales on the two panels. The dotted line represents a perfect model fit, the solid line represents the actual model fit.
Mid-infra-red reflectance spectra have been measured for all soils for which the sorption has measured and we are now testing different machine learning approaches to determine whether MIR spectra can be used more accurately estimate sorption coefficients than wet chemistry properties. This should enable prediction of sorption coefficients, and therefore estimates of herbicide bioavailability, in different soil types through a cheap and rapid MIR scan.
Analysis of field soils
Pre-sowing samples from Southern region sites
Four paddocks (two from SA and two from Vic) were analysed for imidazolinone herbicide residues. Although imidazolinone herbicide residues were detected in all paddocks (Table 3), there was significant variation in concentrations between grid samples within each paddock. For example, at the Lawloit site, two samples were less than the limit of detection (<1.0ng/g), one sample contained 3.6ng/g imazapic and one sample contained 5.4ng/g. Such variation within a paddock has been frequently observed in previous projects and it is important to bear in mind when interpreting soil analysis results.
Table 3. Imazapic toxicity thresholds for 30% root length reductions (ED30) for crops grown in sand. *Average of two individual dose-response experiments.
Site | Soil properties | Imazapyr | Imazamox | Imazapic |
|---|---|---|---|---|
Limit of detection (LOD) (ng/g) | 1.0 | 1.0 | 1.0 | |
Elliston, SA | TBC | 1.9 (<LOD-2.0) | <LOD | 2.6 (<LOD-3.2) |
Koongawa, SA | TBC | <LOD | <LOD | 1.4 (<LOD-2.0) |
Jil Jil, Vic | TBC | <LOD | 2.4 (<LOD-2.4) | <LOD |
Lawloit, Vic | TBC | 2.0 (<LOD-2.4) | <LOD | 4.5 (<LOD-5.4) |
Despite detection of these imi-herbicide residues, none of the samples were predicted to significantly exceed toxicity thresholds for sensitive crops (i.e. wheat, barley, canola) – however some samples were around the 20% shoot biomass toxicity thresholds for wheat seedlings. This included one grid sample at Koongawa (2.0 ng/g imazapyr) and one sample at Lawloit (2.4 ng/g imazapyr). The samples from Lawloit and Elliston also contained residues of both imazapyr and imazapic and although none of the samples from these sites contained imazapic above the preliminary toxicity thresholds for wheat, it is likely that they could have an additive effect when combined with imazapyr residues.
Conclusion
- Dose-response thresholds have been developed for eight winter crops exposed to imazapyr residues in soil, and three winter and three summer crops exposed to imazapic residues. Shoot biomass reduction of 20% for sensitive crops (wheat, barley, canola) occurred at 1ng/g imazapyr or less in pure sand (maximum bioavailability), but ranged from 1.3-6.2ng/g imazapyr in two soil types (sand and loam) tested in glasshouse experiments. Root length reduction of 30% for imazapic occurred in sorghum at 3.7 ng/g in sand, with threshold of >10ng/g for other crops.
- Sorption of imidazolinone herbicides is low in most soil types, especially alkaline soils, and can be predicted if the soil pH, OC content and clay content is known. Low sorption indicates that these herbicides are mobile and relatively bioavailable.
- There is often significant variation in herbicide residue concentrations and soil properties affecting bioavailability across a single paddock. If growers wish to send soil samples for residue analysis, multiple samples from a paddock should be submitted for analysis and should try to account for variations in soil properties/topology across the paddock. Thresholds presented in this paper are relevant for samples taken from a 0-10 cm depth.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. The Southern region component of the project has been supported by the Cooperative Research Centre for High Performance Soils whose activities are funded by the Australian Government's Cooperative Research Centre Program, as part of the ‘Developing knowledge and tools to better manage herbicide residues in soil’ project (4.2.001). More information at Soil CRC Current Projects. Thanks to Lee Kearney, Scott Petty and Kelvin Spann for technical support
Soil CRC project 18-2.P.4.3
Useful resources
Herbicide residues in soil what is the scale and significance
References
Fleming, J., McNee, T., Cook, T. and Mannning, B. 2012 Weed control in summer crops 2012-13. New South Wales Department of Primary Industries, Orange, NSW.
Gianelli, V.R., Bedmar, F. and Costa, J.L., 2014. Persistence and sorption of imazapyr in three Argentinean soils. Environmental toxicology and chemistry, 33(1), pp.29-34.
Llewellyn, R., Ronning, D., Ouzman, J., Walker, S., Mayfield, A. and Clarke, M., 2016. Impact of weeds on Australian grain production: the cost of weeds to Australian grain growers and the adoption of weed management and tillage practices. Report for Grains Research & Development Corporation, Canberra, ACT.
Porfiri, C., Montoya, J.C., Koskinen, W.C. and Azcarate, M.P., 2015. Adsorption and transport of imazapyr through intact soil columns taken from two soils under two tillage systems. Geoderma, 251, pp.1-9.
Wehtje, G., Dickens, R., Wilcut, J.W. and Hajek, B.F., 1987. Sorption and mobility of sulfometuron and imazapyr in five Alabama soils. Weed Science, pp.858-864.
Contact details
Dr Michael Rose
NSW Department of Primary Industies, 1243 Bruxner Highway, Wollongbar, NSW 2477
0266261117
mick.rose@dpi.nsw.gov.au
@NSWDPI_Soils
GRDC Project Code: US00084, UOS1703-002RTX,
