Grain storage, what’s new: managing large flat-bottom silos; grain protectants; storing pulses; resistance update
Grain storage, what’s new: managing large flat-bottom silos; grain protectants; storing pulses; resistance update
Author: Philip Burrill, Greg Daglish, Manoj Nayak, Raj Jagadeesan (Department of Agriculture and Fisheries, Queensland) | Date: 10 Aug 2021
Take home message
- Regular monthly monitoring of stored grain and keeping records for both small and large capacity silos is critical for successful grain quality results and storage pest control
- Carefully check the design and build quality of any monitoring equipment before purchase and instalment in silos. Grain temperature and equilibrium relative humidity (ERH) information is valuable but requires robust sensors, carefully placed
- Monitoring of fumigation gas levels in large silos and storages such as grain bunkers and sheds helps to check if grain pests have had the required dose over time to achieve effective control.
- Talk with potential grain buyers and seek advice prior to applying grain protectant treatments. Each year restrictions change with respect to acceptance of insecticide residues in both domestic and export markets
- Storage pests can develop resistance to fumigants and grain protectants. Growers should be alert to this threat and ensure that phosphine fumigations are conducted to the highest standard and choose effective grain protectant combinations
- Effective management of insect pests of stored pulses is possible using an integrated approach: pest monitoring, aeration cooling and phosphine fumigation.
Managing large flat-bottom silos – grain quality & pests
- There has been widespread investment in large flat-bottom silos by many grain growers as part of on-farm storage facilities. While large capacity (600 – 2000 tonnes), flat-bottom silos are a cost-effective on-farm storage method, they currently have a significant downside. The silo design makes it difficult to carry out effective checks for storage pests and storage conditions, such as grain temperature and moisture content.
- Regular monitoring is critical to ensure grain quality is maintained and pests are controlled in a timely manner. In addition, an efficient method for monitoring gas levels at a number of locations in a large silo during a fumigation would also be beneficial.
- While there are grain storage monitoring systems available from the USA and Canada, most have design issues that currently make them unsuitable for the majority of silos / storages in Australia.
 Figure 1. A farm storage facility with ten large flat-bottom silos, each with 1500 tonnes capacity (photo DAF Qld)
Figure 1. A farm storage facility with ten large flat-bottom silos, each with 1500 tonnes capacity (photo DAF Qld)

Figure 2. Cable or canister monitoring systems can be located inside a silo to monitor grain temperature, humidity or gases
Things to consider when assessing storage monitoring systems
- Measuring both grain temperature and equilibrium relative humidity (ERH) is valuable as it provides information on storage conditions, grain moisture content and providing insight as to how active insect pests are, if present
- Tests following phosphine fumigation have shown that sensors inside a silo designed to measure humidity in grain can be permanently damaged by phosphine gas during a standard fumigation
- Sensor location inside a silo is critical. If sensors are too close to silo walls, readings may be influenced by excessive grain trash or external temperatures i.e. sun or shade on walls
- Some sensors may be difficult to install in silos and to access later if they require maintenance
- Grain storage sensors and cables are in a hostile environment with dust, heat, moisture and significant physical stresses when the silo is filled, emptied and as grain settles during storage
- Sensor’s build quality, lifespan and long-term accuracy will be important for each parameter i.e. grain temperature, humidity and in some cases gas concentration measurements
- Reliable communication of data between the internal storage sensors and external reading / recording devices will be required.
Pest detection and fumigation
Timely pest detection in stored grain is critical to ensure grain is ready for sale, meeting the ‘nil live stored grain insects’ delivery standard. Early detection of pests is also important to ensure significant grain losses and grain quality damage does not occur.
While it is relatively easy to sample grain and check for pests in small cone-based silos, it is difficult to check for storage pests in large flat-bottom silos with limited access to sample grain at the base and top of silos. Currently there appears to be very few commercial products offered to resolve this pest detection issue for large silos.
Unfortunately, this can lead to the practice of ‘calendar based’ regular fumigations of large silos. Silos are fumigated with no knowledge of either pest numbers or pest species present. Any grain stored for 12 – 18 months plus, may then receive multiple fumigations, some of which may not have been required. This is when the risk of development of insect pest resistance to fumigation products like phosphine increases substantially.
Measuring gas concentrations during fumigation at a number of locations inside large silos or other large capacity storage types like grain bunkers or sheds is important. For any fumigation to be effective at controlling storage pests, the insects need to be exposed to a given gas concentration (‘C’), for a specified length of time ‘(T)’.
If this ‘C x T’ exposure requirement is not met throughout all parts of the storage during the fumigation, survival of various insect life cycle stages (eggs, larvae, pupae, adults) is likely. Live adult insect pests will quickly reappear in the grain within days or weeks.
Along with not killing the pests, poor fumigation can also lead to selection and development of resistant insect populations.
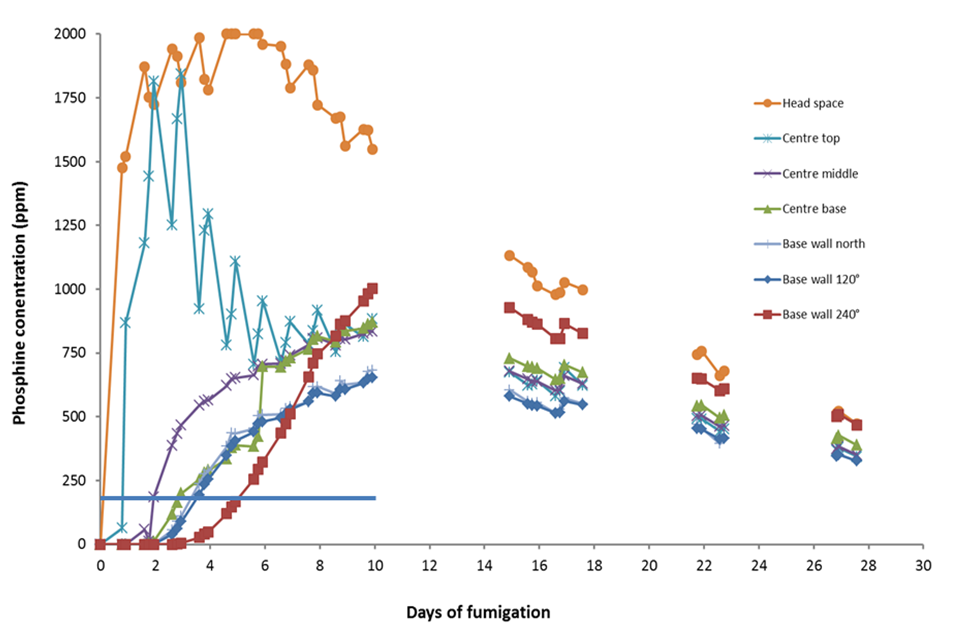 Figure 4. Phosphine gas concentrations at 7 locations in a silo fumigating 1420 t of wheat. Phosphine blankets were placed in the silo headspace with no gas recirculation. It took 5 days before all grain at the silo base reached at least 200 ppm gas concentration.
Figure 4. Phosphine gas concentrations at 7 locations in a silo fumigating 1420 t of wheat. Phosphine blankets were placed in the silo headspace with no gas recirculation. It took 5 days before all grain at the silo base reached at least 200 ppm gas concentration.
Potential future ideas for grain & pest monitoring equipment
- Development of grain storage humidity sensors that are not damaged by phosphine fumigations would add significant value to the current commercial grain moisture cables or canisters (e.g. OPI® moisture cable)
- Developing a new storage cable designed to included both temperature sensors plus 3 or 4 air sample lines to sample air at various heights within the storage could provide multiple measurement functions e.g., equilibrium relative humidity (ERH), fumigation gas levels and detection of insect pest pheromones
- Fitting small ports through the silo wall next to silo ladder landings provides access for 1.0 to 1.5 meter-long grain probes. These 6 mm diameter stainless steel probes are currently available (Graintec Scientific™) to measure grain temperature and to take air samples for a range of functions.
Grain protectant treatments update
Warning: Grain protectant notes below do not apply to the grains industry in Western Australia where their use is restricted. In all cases, product labels are to be used to determine correct use patterns.
Grain protectant sprays – a useful tool for storage management
Strategic use of grain protectant insecticides is only one of five key strategies to maintain grain quality and achieve reliable pest control results. Combined, they form the foundation of successful grain storage. Good results with on-farm grain storage is crucial in the long term for a producer to build a reputation as a reliable supplier of quality grain with no pest problems. Key aspects of successful grain storage are:
- Aeration: correctly designed and managed, it provides cool grain temperatures and uniform grain moisture conditions. Aeration reduces problems with moulds and insect pests in storage, plus maintains grain quality attributes such as germination, pulse seed colour, oil quality and flour quality.
- Hygiene: is crucial in keeping background pest numbers to a minimum and reducing the risk of grain contamination.
- Monitoring: monthly checking of grain in storage for insect pests (sieving / trapping) as well as checking grain quality and temperature. Record these details, including any grain treatments applied.
- Fumigation: in Australia we now only have gases (fumigation) to deal with insect pest infestations in stored grain. To achieve effective fumigations the storage/silo must be sealable – gas-tight (AS2628) to hold the gas concentration for the required time.
- Grain protectants: used on specific parcels of grain like planting seed held on farm, or bulk grain where potential grain buyers have agreed to its use in accordance with the currently registered label, grain protectant sprays provide another line of defence against storage pests in specific situations.
Use of grain protectants on cereal grains for both domestic and export markets is becoming increasingly difficult. Acceptance of insecticide chemical residues on grain, even at extremely low levels, has changed significantly in the last few years. It is important that growers always check with potential grain buyers before using grain protectants.
The Manildra Group™ is a recent example of a large tonnage, Australian wheat buyer who has been required to move to pesticide residue free (PRF) grain due to a number of European and Asian countries banning residues of commonly used grain protectant chemicals such as chlorpyrifos-methyl.
When to use grain protectants
- Typically, protectant sprays are applied to clean cereal grain at harvest time as grain is augered into storages, providing storage pest protection for 3 to 9 months. Protectants are effective at controlling insects as they invade or emerge from eggs within grain during storage
- Grain protectant sprays are not to be used to disinfest grain. When live storage pest insects are detected, fumigation in a sealable silo is required for effective control
- With many domestic and export markets seeking grain supplies which are ‘pesticide residue free’ (PRF), always talk to potential grain buyers / traders prior to applying grain protectant sprays
- The general rule is that NO protectant sprays can be applied to pulses and oilseeds. Always check labels.
- Planting seed held on-farm – wheat, barley, oats
- Grain held for an extended time in non-sealable storages (not suited for fumigation) and the grain buyer has agreed to grain protectant use that is in line with directions for use on the registered product label.
- Grain held on-farm as feed for livestock with agreement from livestock agent or buyer and is in line with directions for use on the registered product label.
Common ‘on-farm’ uses for grain protectants (always read and follow directions for use on the registered label before using)
- Planting seed held on-farm – wheat, barley, oats
- Grain held for an extended time in non-sealable storages (not suited for fumigation) and the grain buyer has agreed to grain protectant use that is in line with directions for use on the registered product label.
- Grain held on-farm as feed for livestock with agreement from livestock agent or buyer and is in line with directions for use on the registered product label.
Grain protectant choices
Examples of two products, which include a partner product, to control the main storage pest species:
1. Conserve® Plus Grain Protector – active ingredient: 100 g/L spinosad, 100 g/L s-methoprene. Used in combination with a compatible organophosphate (OP) product such as chlorpyrifos-methyl (e.g. Diplomat 500 EC), or fenitrothion
For label and details on product use, see: Conserve® Plus Grain Protectant | Corteva Agriscience
Key recommendations
- Always add the OP partner to Conserve Plus so rice and maize weevil (Sitophilus spp.) is controlled.
- Spray equipment calibration and application care are critical to achieve correct dose and uniform coverage on grain.
- If treated grain is exposed to light, for example a semi open grain shed, cover the grain surface with a tarp or 80 - 90% shade cloth. Sunlight breaks down Conserve Plus over time
- Take care to read notes on the web site (above) and seek advice when purchasing Conserve Plus.
2. K-Obiol® EC Combi, synergised grain protectant – active ingredient: 50 g/L deltamethrin,
400 g/L piperonyl butoxide. Used in combination with an organophosphate (OP) partner e.g. chlorpyrifos-methyl or fenitrothion.
For label and details on product use, click here.
Key recommendations
- To control rice, maize and granary weevils (Sitophilus spp.) add a recommended partner (e.g. OP) to the tank mix.
- To ensure effective pest control and that MRLs are not exceeded, calibrate spray equipment and aim for even treatment / coverage on grain.
- Grower users are required to complete a brief (approx. 60 minutes) online training course to be an ‘approved user’ prior to purchase of K-Obiol® EC Combi. See above web site.
Insecticide resistance management
If possible, aim to rotate chemical active ingredients for storage pest control at your storage facility. As an example, two years use of Conserve Plus™ product combination, followed by one or two years of K-Obiol® EC Combi.
Please read and follow all label recommendations and ensure that the product is registered for use in your state prior to application of any product.
Application of grain protectants
Grain protectant application requires care to achieve the correct dose and uniform grain coverage. This leads to effective pest control results and ensures MRL’s are not exceeded. See Figure 5 below.
- Auger’s grain transfer rate. Ensure you have good understanding of the grain flow rate (tonnes per hour) for the particular height the auger will be operating at
- Calibrate your spray application unit with water and check appropriate nozzles and spray pressure are used to achieve the required application of 1 litre of spray mixture per tonne of grain.
Figure 5. Spray application equipment designed for good coverage by applying treatment at two points in the auger
Grain pest resistance update – phosphine and grain protectants
Phosphine resistance
Phosphine fumigation is the most commonly used method used by growers and other grain handlers to disinfest grain. Over recent decades there has been a steady increase in both the prevalence and strength of phosphine resistance in various insect pests of stored grain. Despite these trends, phosphine fumigation remains effective against resistant pests except for one species.
The strongest level of phosphine resistance occurs in the rusty grain beetle (Cryptolestes ferrugineus) . This tiny beetle is one of several species of flat grain beetles (Cryptolestes species) found on farms (Figure 6). Although not normally the most common pest on farms, the presence of strongly resistant rusty grain beetles will threaten fumigation success, even in well-sealed silos.
GRDC has supported phosphine resistance monitoring for many years, as well as research on managing resistant pests. Our testing procedure allows us to categorise populations as susceptible, weakly resistant or strongly resistant, based on the presence of survivors in laboratory tests. Table 1 shows the results of recent resistance testing of pest populations collected from farms located in the GRDC northern region. The results show that phosphine resistance is common. Concerningly, half of the rusty grain beetle populations were categorised as strongly resistant.
Table 1. Phosphine resistance testing of grain beetle populations collected from farms in the GRDC northern region (2020-2021).
Pests | Number of populations | ||
|---|---|---|---|
Susceptible | Weak resistance | Strong resistance | |
Lesser grain borer (Rhyzopertha dominica) | 0 | 43 | 21 |
Rust-red flour beetle (Tribolium castaneum) | 12 | 49 | 15 |
Rice weevil (Sitophilus oryzae) | 1 | 10 | 0 |
Saw-toothed grain beetle (Oryzaephilus surinamensis) | 0 | 6 | 0 |
Flat grain beetles (Cryptolestes species)* | 0 | 12 | 6 |
* There are several Cryptolestes species, but only rusty grain beetle (Cryptolestes ferrugineus) has been shown to develop strong resistance.
Two phosphine fumigation trials conducted at Warwick in February 2017 show how difficult it is to control strongly resistant rusty grain beetles compared with strongly resistant populations of other pests. Silos containing wheat were fumigated according to the registered label for fumigations using aluminium phosphide tablets, with one trial lasting 7 days and the other 10 days. Cages of pests were placed in the silo at three depths (top, middle and bottom) to evaluate fumigation success. The species tested were the rusty grain beetle, the lesser grain borer, the rust-red flour beetle, the rice weevil and the saw-toothed grain beetle. The cages contained all life stages (eggs, larvae, pupae and adults) because phosphine tolerance can vary between life stages.
All species except for the rusty grain beetle were controlled in both the standard 7-day fumigation and the extended 10-day fumigation. The resistant rusty grain beetles were much harder to control (Table 2). As is often observed, the highest average phosphine concentrations were in the upper part of the silo and the lowest in the lower part of the silo. In the 7-day fumigation, there was a high level of population suppression (99.7%) of the rusty grain beetle in the top and middle cages but suppression was negligible (8.6%) in the bottom cages. Efficacy was better in the extended 10-day fumigation with high levels of suppression (99.4-100%) at all three levels of the silo. Similar average phosphine concentrations were achieved in the two fumigations but the extra 3 days made the 10-day fumigation much more effective against the strongly resistant rusty grain beetles.
Table 2. Phosphine efficacy against strongly phosphine resistant infestations of rusty grain beetle (Cryptolestes ferrugineus) in sealable silos (46.5 cubic metre volume)
Measurement | Cage location in silo | Fumigation trial | |
|---|---|---|---|
7-day | 10-day | ||
Average concentration (ppm) | Top | 1368 | 1232 |
Middle | 936 | 899 | |
Bottom | 577 | 628 | |
Infestation suppression (%) | Top | 99.7 | 100 |
Middle | 99.7 | 100 | |
Bottom | 8.6 | 99.4 | |
 Figure 6. Flat grain beetles, Cryptolestes spp.
Figure 6. Flat grain beetles, Cryptolestes spp.
Grain protectant resistance
Grain protectant insecticides are used by many growers and other grain handlers where storage structures cannot be sealed well enough for fumigation. There has been a long history of stored grain pests developing resistance to protectants. GRDC has supported protectant resistance monitoring for many years, as well as research on managing resistant pests.
A study is under way testing grain pests to some commonly used protectants. Table 3 shows some preliminary results. As mentioned earlier, no single grain protectant will control all species or all resistant types within a species, so combinations of two or three protectants are needed. This is reenforced by these results.
Chlorpyrifos-methyl is an old organophosphate protectant that targets rust-red flour beetles and rice weevils. There was no evidence of resistance in either species.
S-methoprene is an insect growth regulator that targets lesser grain borers and several other pests. Resistance was first detected in lesser grain borers in the 1990s, so its lack of control of the lesser grain borer populations tested is not surprising. The poor control of rice weevils is expected – not because of resistance but an innate tolerance. S- methoprene controlled about half of the rust-red flour beetle populations and this could be because of resistance or a degree of innate tolerance.
Spinosad was registered for control of lesser grain borers but combined treatments were tested because spinosad is no longer available as a stand-alone treatment. The results for the spinosad combination treatments show how combinations can overcome various protectant resistances to maximise the breadth of pest coverage.
Table 3. Number of populations of lesser grain borer (Rhyzopertha dominica; LGB), rust-red flour beetle (Tribolium castaneum; RRFB) and rice weevil (Sitophilus oryzae; RW) controlled by grain protectant treatments.
Treatment (ppm) | Pest | ||
|---|---|---|---|
Number of populations tested for each pest (n) | LGB (n =7) | RRFB (n = 12) | RW (n = 5) |
Chlorpyrifos-methyl (10) | 0 | 12 | 5 |
S-methoprene (0.6) | 0 | 5 | 0 |
S-methoprene (1) + spinosad (1) | 7 | 12 | 0 |
Chlorpyrifos-methyl (10) + s-methoprene (1) + spinosad (1) | 7 | 12 | 5 |
Successful storage of pulses
Managing pests of stored pulses
The pulse industry is growing rapidly in the GRDC northern region and the Queensland Government has recently supported a project on effective management practices for pests of stored pulses, especially mungbeans and chickpeas. A lot of research has been done overseas but this has limited relevance to Australia, because of the focus on cowpeas and subsistence agriculture.
Recent farm sampling in the GRDC northern region shows that infested mungbeans and chickpeas can contain pests normally associated with stored cereals, as well as the cowpea weevil (Callosobruchus maculatus, also known as bruchids), which is the major pest of stored pulses (Table 4). The cereal pests are probably not causing much damage to pulses, but their presence will negate their insect-free status. Cowpea weevil adults do not feed but the larvae cause major damage as they develop inside individual pulse seeds, and newly developed adults emerge from large exit holes in the pulse seeds (Figure 7).
Cowpea weevils are good fliers. Although the cowpea weevil is a serious pest of stored pulses, overseas studies show that it can infest the cowpea crop before harvest. For cowpeas at least and potentially other pulses, infestation can be caried over from the field and into storages. Pre-harvest infestation is rare in most pests of stored cereals, except for the maize weevil (Sitophilus zeamais).
Table 4. Detections of insect pests in infested mungbeans and chickpeas collected from farms in the GRDC northern region.
Pests | Mungbeans (8 samples) | Chickpeas (27 samples) |
|---|---|---|
Cowpea weevil (Callosobruchus maculatus) | 5 | 4 |
Lesser grain borer (Rhyzopertha dominica) | 5 | 14 |
Rust-red flour beetle (Tribolium castaneum) | 6 | 26 |
Rice weevil (Sitophilus oryzae) | 0 | 6 |
Saw-toothed grain beetle (Oryzaephilus surinamensis) | 0 | 2 |
Flat grain beetles (Cryptolestes species) | 0 | 4 |
 Figure 7. The distinctive large white eggs and round exit holes of the cowpea weevil (Callosobruchus maculatus) on mungbeans. The eggs are white because the larvae have hatched and burrowed into the mungbeans. (Source: QDAF)
Figure 7. The distinctive large white eggs and round exit holes of the cowpea weevil (Callosobruchus maculatus) on mungbeans. The eggs are white because the larvae have hatched and burrowed into the mungbeans. (Source: QDAF)
Pest monitoring
As with stored cereals, regular monthly inspections and sampling of stored pulses is essential to check both grain quality and for the presence of pests. Sieving of pulse samples from the top and bottom of storages is useful for early detection, as is trapping with probe or pitfall traps placed in the grain surface. Newly laid cowpea weevil eggs are hard to see but are white after the larvae hatch and burrow into the seeds. Adult cowpea weevils are easy to see, as are the large exit holes in the seeds.
Aeration cooling
Population growth of insects is strongly dependent on temperature and aeration cooling has long been promoted as a chemical-free means of reducing infestation levels in stored cereals. Similarly, aeration cooling can be used to reduce infestation levels in cowpea weevils. Figures 8 and 9 show the impact of temperature on generation time and multiplication rate per generation in this pest. The temperature at which population growth was zero was estimated to be 17˚C using these results.
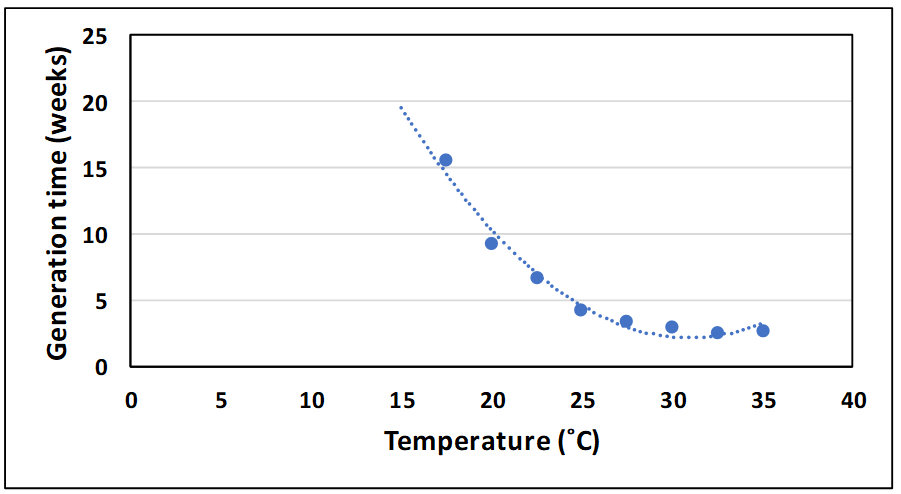 Figure 8. Effect of temperature on generation time of the cowpea weevil (Callosobruchus maculatus) in mungbean.
Figure 8. Effect of temperature on generation time of the cowpea weevil (Callosobruchus maculatus) in mungbean.
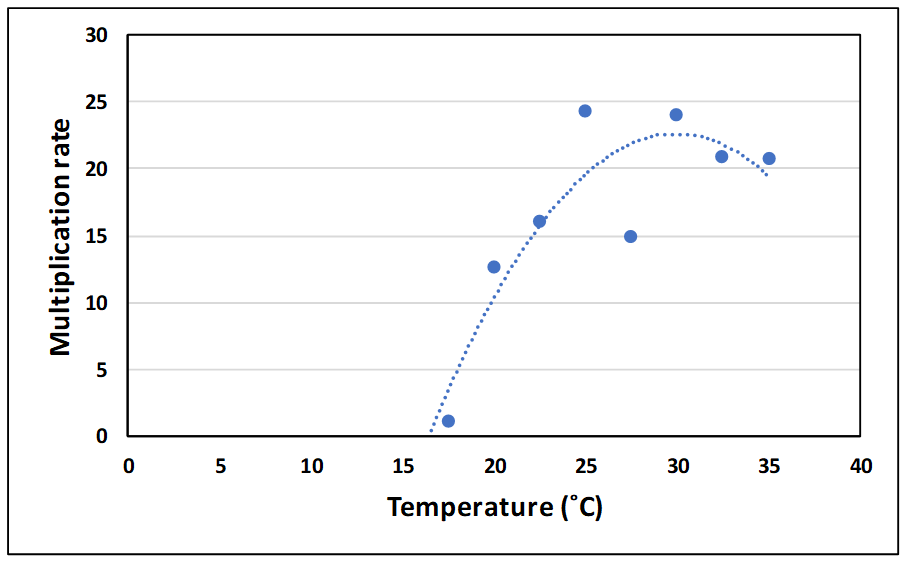 Figure 9. Effect of temperature on multiplication rate of the cowpea weevil (Callosobruchus maculatus) in mungbean.
Figure 9. Effect of temperature on multiplication rate of the cowpea weevil (Callosobruchus maculatus) in mungbean.
Phosphine fumigation
Phosphine fumigation can be used by growers and other grain handlers to disinfest pulses and there is no evidence to date of resistance in the cowpea weevil. Because of a lack of information on the efficacy of phosphine against cowpea weevils, laboratory fumigations and silo-scale fumigations were conducted.
Phosphine efficacy against other pests is known to be affected by concentration and time. Therefore, this was investigated in the laboratory using fixed concentrations and exposure periods. Cages of pests were placed in the silo at several depths to evaluate fumigation success against cowpea weevil infestations. Mixed-age infestations containing all life stages (eggs, larvae, pupae and adults) were used because phosphine tolerance in other species can vary between life stages.
Table 5 shows that both higher phosphine concentrations and longer exposure periods increase phosphine efficacy against cowpea weevils. Some variation in tolerance to phosphine was evident between different test populations, but concentrations of 360-720 ppm for 7 days would result in high levels of infestation suppression.
Table 5. Phosphine efficacy against infestations of cowpea weevils (Callosobruchus maculatus) in laboratory fumigations.
Exposure (days) | Concentration (ppm) | Infestation suppression (%) | ||
|---|---|---|---|---|
Mungbeans | Chickpeas (Desi) | Chickpeas (Kabuli) | ||
1 | 360 | 54.7-86.8 | 87.8-99.2 | 96.8-100 |
720 | 69.0-88.3 | 98.1-99.0 | 100 | |
4 | 360 | 90.0-99.6 | 85.5-100 | 100 |
720 | 99.8-100 | 99.8-100 | 97.1-100 | |
7 | 360 | 99.8-100 | 100 | 100 |
720 | 99.5-99.7 | 100 | 100 | |
Two phosphine fumigation trials were conducted at Warwick on mungbeans (March 2019) and chickpeas (March 2021). Silos containing mungbeans or chickpeas were fumigated according to the registered label for fumigations using aluminium phosphide tablets. Cages were placed in the silo at several depths to evaluate fumigation success against cowpea weevil infestations. The cages contained all life stages (eggs, larvae, pupae and adults) because phosphine tolerance in other species can vary between life stages.
The results show that phosphine fumigation conducted according to the registered label resulted in high levels of suppression of cowpea weevil infestations (Table 6).
Table 6. Phosphine efficacy against infestations of cowpea weevils (Callosobruchus maculatus) in 7-day fumigations of sealable silos (11.1 cubic metre volume)
Measurement | Cage location in silo | Fumigation trial | |
|---|---|---|---|
Mungbeans | Chickpeas | ||
Average concentration (ppm) | Top | 1040 | 1187 |
Middle | 945 | 1199 | |
Bottom | 909 | 1213 | |
Infestation suppression (%)* | Top | 99.8-100 | 100 |
Middle | 99.9-100 | 100 | |
Bottom | 100 | 100 | |
Diatomaceous earth (DE)
Diatomaceous earth (DE) can be used as a structural treatment as part of storage hygiene. Treating empty storages will control cereal pests sheltering in cracks and crevices. Field trials on structural treatments are difficult so DE efficacy against the cowpea weevil was investigated in the laboratory.
Concrete and stainless-steel surfaces were treated with a commercial DE product (Dryacide®) at the rate of 2 g/m2 and held at either 25˚C and 55% RH or 30˚C and 70% RH. Efficacy was determined after 0 and 3 months by adding adults and monitoring mortality for up to 7 days. High levels of mortality occurred on treated concrete and steel within 7 days of exposure (Table 7).
Table 7. Mortality of cowpea weevils (Callosobruchus maculatus) exposed to surfaces treated with diatomaceous earth (DE) at 2 g/m2.
Surface | Assessment | Storage conditions | |
|---|---|---|---|
25˚C and 55% RH | 30˚C and 70% RH | ||
Concrete | 0 months | 100% by 2 days | 99.2% by 7 days |
3 months | 100% by 2 days | 99.2% by 7 days | |
Steel | 0 months | 100% by 2 days | 100% by 7 days |
3 months | 92.9% by 7 days | 97.9% by 7 days | |
A new publication on pulse storage will be released in the second half of 2021. The publication “Best management practices for storage of pulses” will be published by Queensland’s Department of Agriculture and Fisheries. It documents further research and management details to add to the pulse storage data included above. It provides the pulse industry in Australia with a valuable pulse storage management reference guide for the future.
Further information
GRDC fact sheet – Vigilant monitoring protects grain assets
GRDC booklet – Fumigating with phosphine other fumigants and controlled atmospheres
GRDC fact sheet – Pressure testing sealable silos
GRDC video – Fumigation recirculation
Corteva agriscience - Conserve Plus™ Grain Protector
BAYER CropScience - K-Obiol® EC Combi
Acknowledgements
The authors acknowledge GRDC support, specifically RD&E projects PRB2011-001SAX and DAQ1906-00RTX, under which a range of research and extension activities was conducted. The authors would also like to thank DAF’s Postharvest Research Team members, GRDC’s national grain storage extension team, along with valued support from growers and other industry collaborators.
Contact details
Philip Burrill
Department of Agriculture and Fisheries, AgriScience Qld.
Hermitage research facility, 604 Yangan Rd, Warwick Qld. 4370
Mb: 0427 696 500
Email: philip.burrill@daf.qld.gov.au
Greg Daglish
Department of Agriculture and Fisheries, AgriScience Qld.
EcoSciences Precinct, Dutton Park, Brisbane
Email: greg.daglish@daf.qld.gov.au
® Registered trademark
TM Trademark
GRDC Project Code: PRB2011-001SAX, DAQ1906-00RTX,

