Nitrogen loss pathways – how much do we lose and in what form under different situations
Nitrogen loss pathways – how much do we lose and in what form under different situations
Author: Graeme Schwenke, NSW DPI | Date: 07 Jun 2022
Take home message
There are many directions (upwards, downwards, sidewards) and forms (gas, liquid, solid) that nitrogen (N) may leave the soil! Overall N losses are often minimal, but there are a range of pathways that can lead to significant reduction of available soil N needed for crop nutrition. Some losses can occur directly after N fertiliser is applied to the soil (e.g., volatilisation), others can occur months or even years after fertiliser is applied (e.g., denitrification). Most losses are driven by environmental conditions, either directly (e.g., rainfall intensity) or through indirect effects on soil microbial communities that conduct many of the component processes. Inherent soil properties can also have major impacts on N losses, particularly in relation to water drainage (e.g., clay, silt and sand content) and pH buffering capacity (e.g., soil organic matter, clay content). Knowing your soil properties and climatic drivers of N losses is key to managing soils to minimise N loss and maximise applied N use efficiency.
Nitrogen pathways
The cycling of nitrogen (N) through the soil, water and air is complex. Figure 1 shows only the overall N-forms and directions of flow, but not the detailed reactions within each conversion of N. There are many solid, liquid (solution) and gaseous forms of N that are interconnected by a variety of processes—some biologically-driven others purely chemical. What diagrams like Figure 1 do not show, are the relative amounts of N present in the different forms, and the different rates of change from one form to another. A range of inherent soil properties, crop growth and environmental conditions (moisture content, temperature, wind speed) all combine to determine whether N will be lost and from which pathway and at what rate. A recently published review (Barton et al. 2022) has summarised data from published Australian research into N losses from dryland grains systems and highlights from this are presented here.
Nitrogen losses
If there were no N losses from fertiliser N applied to the soil-plant system, then the amount of N taken up by the crop or left in the soil at harvest would equal that which was put there at the start of the season, plus that mineralised from soil organic matter during the season. We know N losses occur because many studies using isotopically-labelled 15N fertiliser were not able to account for all of the 15N applied when measurements of plant and soil were conducted at harvest. From a large number of Australian cropping studies, an average of 28% of the 15N applied to a crop was not found at the end of the crop (range: 0–94%) (Barton et al. 2022). There are a range of potential loss pathways that can contribute to the overall loss figure. Volatilisation, leaching and runoff can be measured directly. Denitrification is usually deduced by difference after measuring the total N loss and the other measurable pathways.
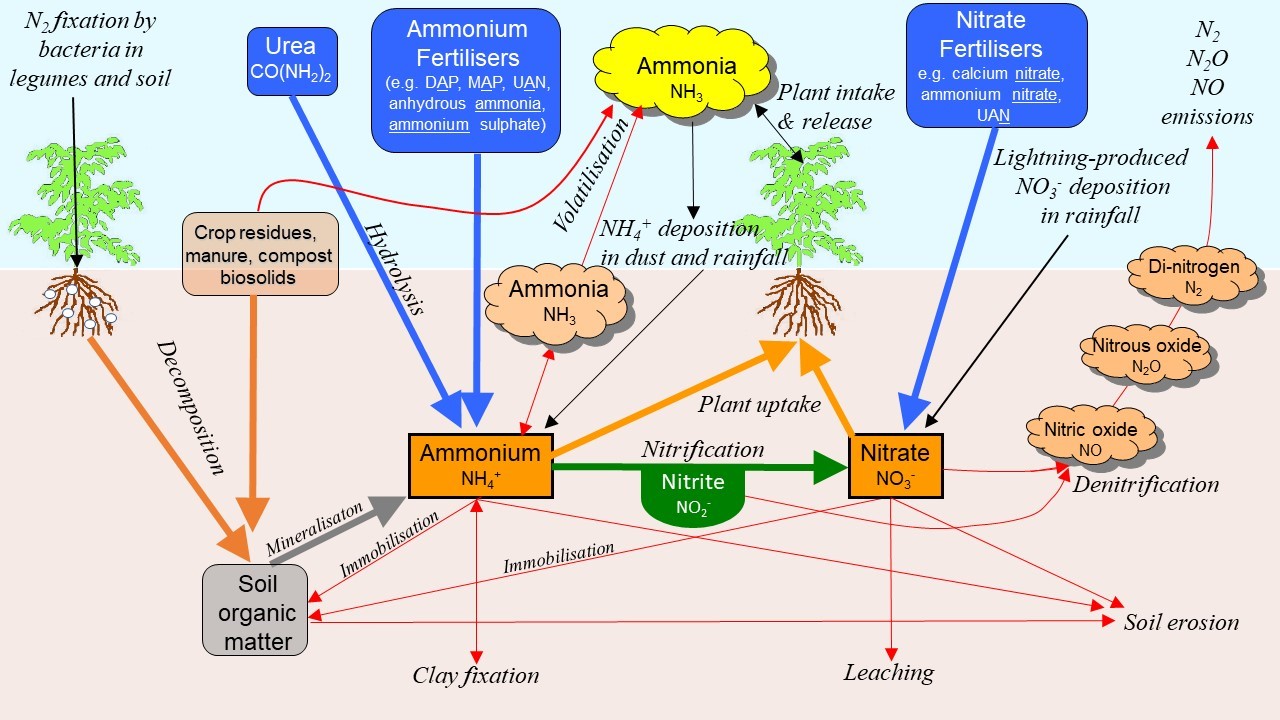
Figure 1. Major pathways of the soil nitrogen cycle.
Volatilisation
The processes involved in volatilisation of ammonia from applied urea fertiliser have been described in detail previously (Schwenke 2022). In summary, urea applied to the soil surface first dissolves into solution, then is hydrolysed (chemically decomposes in combination with water) into ammonium and bicarbonate ions with the help of the urease enzyme. The hydrolysis reaction creates a temporary zone of highly alkaline pH around the former urea granule. Ammonium ions in the soil solution are in equilibrium with ammonia molecules, but the proportion of ammonia increases as pH rises above neutral (pH >7). If the soil can buffer the changes in pH due to hydrolysis, then the proportion of ammonia will remain low, whereas soils with low pH buffering capacity will see a marked pH increase and more ammonia will be present. If this ammonia is near to the soil surface, it can come out of solution as a gas and be lost from the soil via ammonia volatilisation.
Ammonia volatilisation can also occur following addition of other ammonium fertilisers to the soil, but only if the soil pH is already alkaline. A special case is where ammonium sulfate is applied to a calcareous soil (with free lime at the surface) or in conjunction with agricultural lime application. In this instance, a direct chemical reaction between the ammonium sulfate and the lime creates a temporary alkaline pH, which can lead to ammonia volatilisation losses. Ammonia may also be lost during the application of anhydrous ammonia into the soil if the application slots are not immediately covered with soil. Soil moisture content at the time of application (not too dry, not too wet) is the key to preventing this loss.
Field-measured volatilisation studies in the Australian grains industry have been sparse and sporadic and have primarily focussed on losses from surface applications of urea—volatilisation from banded or incorporated N fertiliser should be negligible in most cases. Reported ammonia volatilisation losses from N applied to Australian cropping soils ranged from 0.1–34% (median of 6.7%) (Barton et al. 2022), which is similar to global averages of 6–19.5%, although the global range extends up to 65% (Ma et al. 2020). Average losses of ammonia from surface application of animal manures tend to be higher (23% global median) (Bouwman et al. 2002), but there is limited Australian data. Denmead et al. (2020) found 12% loss during summer grazing of pasture, but only 1% during winter.
Ammonia volatilisation losses typically occur over a period of 1–2 weeks after application but can be prolonged by dry weather or cut short by wet weather. Ammonia volatilisation risk is higher where urea is applied to a wet soil that is drying, than to a dry soil that is rained on. A wet soil dissolves the urea and commences hydrolysis but does not move the ammonium into the soil where greater soil volume helps buffer the change in pH. In contrast, spread urea may sit undissolved on a dry soil until rainfall dissolves and, if in sufficient intensity, washes the dissolved urea down into the soil. Clay in soils helps to absorb ammonium on the cation exchange surfaces and reduce the pool of ammonium/ammonia available to be lost, as well as contributing to pH buffering capacity. Low clay soils with low organic matter have poor pH buffering capacity and are more at risk of ammonia being lost. The risk of volatilisation loss diminishes with time as the pH returns to normal soil pH and the ammonium is nitrified to nitrate or taken up by plants.
An often overlooked N loss pathway from cropping systems is that of volatilisation from the crop itself. While plants can ‘absorb’ ammonia from the air they can also lose N in various volatile forms (ammonia, dinitrogen, nitric oxide, nitrogen dioxide, nitrous oxide and various amines). Overseas studies have shown leaves can lose ammonia from their stomata both after fertiliser application and after anthesis, during grain filling (Harper et al. 1987; Yang et al. 2019). This can happen when the concentration of ammonia within the leaf cells is greater than that in the air surrounding the leaf, thus establishing a gradient for diffusion of ammonia out of the plant. The provision of excess N after anthesis when the plant is translocating N from leaves and stems into grain is therefore a potential cause of such loss. The reverse is also true: where ammonia emissions from the soil increase the concentration in air surrounding the leaves, the plant will absorb the ammonia, thus reducing the outright loss of N to the atmosphere. Ammonia also evolves from plant matter during leaf senescence, residue decomposition or stubble burning, with the amount evolved linked to the N content in the residues (Janzen and McGinn 1991; de Ruijter et al. 2010). Incorporating residues into the soil prevents such losses. There is little Australian data available (Farquhar et al. 1983).
Nitrification
Nitrification is the conversion of ammonium to nitrate by several groups of soil bacteria. As with most N pathways, this conversion involves multiple steps and intermediate N compounds, some of which are gaseous: nitric oxide (NO) and nitrous oxide (N2O). Some of these gases can leak out of the soil during this process and are then lost to the atmosphere. In drier soils, more is lost as NO, whereas more is lost as N2O from wetter soils. In most instances, the total amount of N lost during the nitrification process is minimal, typically <1% of applied N. As both of these gases are also emitted during the denitrification process, it is difficult to separate measured emissions of either gas from these two major source pathways.
Denitrification
Denitrification is the multi-step reduction of nitrate (NO3-) to nitrite (NO2-) then nitric oxide (NO), nitrous oxide (N2O) and finally dinitrogen (N2). This reduction process is carried out by bacterial and/or fungal organisms in lieu of oxygen (O2) being available as an electron acceptor in the formation of ATP. These organisms also need a supply of labile carbon (C) as a food source. Therefore, denitrification can be a significant loss pathway in poorly drained or flooded soils where more than 70% of the soil pores are filled with water thus limiting diffusion of air into the pores. To a lesser extent, it can also occur in drier soils—micro-pores within soil aggregates can be anaerobic even when the inter-aggregate pores have adequate air. As there are several gases produced during denitrification—typically the main one is dinitrogen (N2)—direct measurement of denitrification is difficult and requires the use of highly enriched 15N isotope-labelled fertiliser. Indirect estimation of denitrification loss is more common, using recovery of applied 15N in the soil and plant at the end of the season, with account taken for other N losses where possible.
Directly measured denitrification losses from Australian dryland cropping soils ranged from 0–54% of applied fertiliser N (median, 28%) (Barton et al. 2022). Greatest losses occurred where N fertiliser was applied to clay soils well before sowing. Similarly, studies of pre-season N fertiliser application ahead of irrigated cotton reported N losses of up to 92% of applied N after above-average rainfall in the fallow period (Humphreys et al. 1990). Globally, denitrification rates from upland cultivated fields had an average loss rate of 0.11 kg N/ha/day with an average overall emission factor of 4.1% loss as a proportion of N applied (Pan et al. 2022). On average, 35% of the gas produced was N2O with the remainder N2, but this varies with soil pH and moisture content. More N2 than N2O is produced in the most water-saturated oxygen-deprived soils, whereas more N2O may be produced than N2 in acidic soils.
Methods to reduce the impact of denitrification losses focus on fertiliser application timing (apply closer to, or within, the growing season) and placement of N fertiliser (apply N deeper into the subsoil below the microbially-active and C-rich topsoil). Prophylactic use of a chemical nitrification inhibitor when pre-applying ammonium or urea N fertilisers has proven highly effective at decreasing denitrification losses (as measured by reduced N2O emissions) in many experiments.
Losses of Nitrous oxide (N2O)
Nitrous oxide (N2O) emissions can come from a range of soil biological and chemical processes, with microbial nitrification and denitrification considered to be the main sources. This gas has received a lions-share of research attention in Australia and globally, as it is a long-lived greenhouse gas with a 100-year warming potential equivalent to 298 times that of carbon dioxide. Its concentration in the atmosphere is increasing and it is also the main ozone-depleting gas in the stratosphere.
The median annual N2O emissions from Australian dryland grain cropping soils across many studies was 0.19 kg N/ha (range: 0–48 kg N/ha) (Barton et al. 2022), which represents a median loss of 0.2% of applied N fertiliser, with losses of up to 1.8% reported. Most losses of N2O from fertiliser addition occur during the early crop stage between fertiliser application and crop N uptake, particularly if intense rainfall leads to waterlogging conditions (denitrification). Otherwise, N2O emissions during a dryland cropping system are typically very low with occasional short-lived spikes following rainfall events (likely to be due to a flush of nitrification). After harvest, crop residue breakdown can also liberate N2O emissions, particularly from crops with higher N contents such as legumes and canola.
The highest cohort of N2O losses reported in Australian grain cropping systems occurred following the waterlogging of newly cultivated crops out of long-term legume-based pastures in the high rainfall zone of southeast Australia.
Mitigation of N2O emissions has been demonstrated through (a) splitting or deferring N fertiliser additions to reduce the amount of nitrate in the soil while the crop is establishing, (b) adding a nitrification inhibitor to the N fertiliser or encapsulating it inside a degradable polymer shell, (c) reduced reliance on N fertilisers by increased adoption of crop/pasture legumes, and (d) carefully timing the removal of pasture legumes to avoid waterlogged N-rich conditions.
Leaching
Soluble forms of N may be removed from the root zone in soils via leaching. Nitrate (NO3-) is considered the most susceptible form for leaching, but organic forms of N may also leach. Reports of NO3- leaching in Australia have focussed on deep sands in WA with losses ranging from 4–72 kg N/ha (Barton et al. 2022), with highest losses where the N lost had mineralised from lupin crop residues. Losses from applied fertiliser were 1.2% of applied N in a southeast Australia study. Leaching of applied fertiliser N during a cropping season is unlikely in medium–heavy cracking clay soils (Vertosols) but long-term studies showed movement of NO3- under zero tillage + stubble burning + N fertiliser applied that was equivalent to 30% of the applied N (Turpin et al. 1998). The results in this experiment were attributed to greater porosity in the zero tillage system, as well as inefficient crop use of available N due to nematodes.
As with denitrification, the most effective mitigation option for leaching of N is not having lots of available soil NO3- during the early crop phase when plant demand for N is low and rainfall may be high.
Runoff/erosion
Water and wind erosion can lead to catastrophically high losses of both organic and inorganic N forms in extreme situations. Available N from organic matter mineralisation and fertiliser addition (along with organic N in soil organic matter) is concentrated at the soil surface—where most soil erosion loss occurs. However, there is little research data on this potentially large N loss pathway.
In wind erosion of cultivated bare soils, studies have shown that the smaller particles (<90µm) are removed in dust and these small particles carry away 16 times more N than was present in the soil it was derived from (Leys and McTainsh 1994). Estimates of dust N content were 0.17% for particles <44 µm in another Australian study (Raupach et al. 1994).
Water erosion can be minimised by maintaining ground cover above a critical threshold, maintain soil structure (e.g., limiting compaction by heavy equipment or stock), and creating contours and vegetated waterways to control water flow. In a central Qld study, sediment loss from a Vertosol under zero tillage was 1.2 t/ha compared to 4 t/ha under conventional tillage practice. The calculated loss of NO3- was 8 kg N/ha, which was two thirds of the total N loss and 20% of the fertiliser N applied (Murphy et al. 2013). In a southern Qld Vertosol under annual winter cropping, soil movement of 61 t/ha/yr under bare summer fallow was reduced to 2.1 t/ha/yr under zero tillage (Freebairn and Wockner 1986).
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
References
Barton L, Hoyle FC, Grace PR, Schwenke GD, Scanlan CA, Armstrong RD, Bell MJ (2022) Chapter One - Soil nitrogen supply and N fertilizer losses from Australian dryland grain cropping systems. In 'Advances in Agronomy. Vol. 174.' Ed. DL Sparks) pp. 1-52. (Academic Press)
Bouwman AF, Boumans LJM, Batjes NH (2002) Estimation of global NH3 volatilization loss from synthetic fertilizers and animal manure applied to arable lands and grasslands. Global Biogeochemical Cycles 16(2), 8-1-8-14.
de Ruijter FJ, Huijsmans JFM, Rutgers B (2010) Ammonia volatilization from crop residues and frozen green manure crops. Atmospheric Environment 44(28), 3362-3368.
Denmead OT, Bai M, Turner D, Li Y, Edis R, Chen D (2020) Ammonia emissions from irrigated pastures on Solonetz in Victoria, Australia. Geoderma Regional 20, e00254.
Farquhar GD, Wetselaar R, Weir B (1983) Gaseous nitrogen losses from plants. In 'Gaseous Loss of Nitrogen from Plant-Soil Systems.' (Eds JR Freney and JR Simpson) pp. 159-180. (Springer Netherlands: Dordrecht)
Freebairn D, Wockner G (1986) A study of soil erosion on Vertisols of the eastern Darling Downs, Queensland. I. Effects of surface conditions on soil movement within contour bay catchments. Soil Research 24(2), 135-158.
Harper LA, Sharpe RR, Langdale GW, Giddens JE (1987) Nitrogen Cycling in a Wheat Crop: Soil, Plant, and Aerial Nitrogen Transport. Agronomy Journal 79(6), 965-973.
Humphreys E, Freney JR, Constable GA, Smith JWB, Lilley D, Rochester IJ (1990) The fate of your N fertilizer. In 'Fifth Australian Cotton Conference: the Australian cotton industry under the microscope. '. pp. 161-164. (Australian Cotton Growers' Research Association: Broadbeach, Qld)
Janzen HH, McGinn SM (1991) Volatile loss of nitrogen during decomposition of legume green manure. Soil biology & biochemistry 23(3), 291-297.
Leys J, McTainsh G (1994) Soil loss and nutrient decline by wind erosion-cause for concern [Mallee, New South Wales]. Australian Journal of Soil and Water Conservation (Australia) 7(3), 30-35.
Ma R, Zou J, Han Z, Yu K, Wu S, Li Z, Liu S, Niu S, Horwath WR, Zhu-Barker X (2020) Global soil-derived ammonia emissions from agricultural nitrogen fertilizer application: A refinement based on regional and crop-specific emission factors. Global Change Biology n/a(n/a).
Murphy T, Dougall C, Burger P, Carroll C (2013) Runoff water quality from dryland cropping on Vertisols in Central Queensland, Australia. Agriculture, Ecosystems & Environment 180, 21-28.
Pan B, Xia L, Lam SK, Wang E, Zhang Y, Mosier A, Chen D (2022) A global synthesis of soil denitrification: Driving factors and mitigation strategies. Agriculture, Ecosystems & Environment 327, 107850.
Raupach M, McTainsh G, Leys J (1994) Estimates of dust mass in recent major Australian dust storms. Australian Journal of Soil and Water Conservation 7(3), 20-24.
Turpin JE, Thompson JP, Waring SA, MacKenzie J (1998) Nitrate and chloride leaching in Vertosols for different tillage and stubble practices in fallow-grain cropping. Australian Journal of Soil Research 36(1), 31-44.
Yang Y, Ni X, Liu B, Tao L, Yu L, Wang Q, Yang Y, Liu J, Wu Y (2019) Measuring field ammonia emissions and canopy ammonia fluxes in agriculture using portable ammonia detector method. Journal of Cleaner Production 216, 542-551.
Contact details
Graeme Schwenke
NSW Department of Primary Industries
Tamworth Agricultural Institute
Calala, NSW
Ph: 02 67631137
Email: graeme.schwenke@dpi.nsw.gov.au
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
GRDC Project Code: UOQ2204-010RTX,
