Fusarium head blight and white grain issues in 2022 wheat and durum crops
Fusarium head blight and white grain issues in 2022 wheat and durum crops
Author: Steven Simpfendorfer (NSW DPI, Tamworth), Brad Baxter (NSW DPI, Wagga Wagga) | Date: 28 Feb 2023
Take home messages
- Detection of Fusarium head blight (FHB) was widespread across eastern Australia in 2022
- White grain disorder (WGD, Eutiarosporella formerly Botryosphaeria) was also confirmed in some areas (mainly southern Qld)
- FHB and WGD can be confused with melanism (false black chaff) and stripe rust head infections. Use NSW DPI pathologists for correct identification
- FHB infection is a function of prolonged high humidity (>80%) during flowering and early grain fill
- FHB causes yield loss (up to 100%) but also potentially downgrading of grain due to production of mycotoxins in affected white or pink grains (deoxynivalenol, DON mainly) which can affect end use depending on the level of infection
- Retaining grain from FHB or WGD affected crops negatively impacts suitability for sowing so grain infection levels should be tested.
Where did it come from?
If caused by Fusarium pseudograminearum, then the Fusarium head blight likely came from basal infection of tillers from Fusarium crown rot. Rain splash transports spores on lower nodes into heads. If caused by Fusarium graminearum,then it likely came from air borne spores produced on maize or sorghum stubble or some grass weeds known to be hosts. It can also host on wheat and barley. However, climatic conditions during flowering through to soft dough are a key factor in disease development. Frequent rainfall, high humidity, and/or heavy dews or fogs that coincide with flowering and early grain fill periods favour infection and development of FHB and WGD. The most favourable conditions for FHB infection are prolonged periods (36-72 hours) of moisture (>80% humidity) and warm temperatures (20-30°C). However, infection does occur at cooler temperatures when high moisture persists for longer than 72 hours.
The abundance of inoculum and weather conditions during flowering determines the severity of FHB. The longer the wheat head stays wet during flowering and early grain development, the greater the chance of infection and increased severity. Early infections may produce spores that are responsible for secondary infections under optimum conditions for disease development, especially if the crop has uneven flowering due to late tillers or a prolonged flowering period due to cooler temperatures or phenology.
There is no information on the relative resistance of Australian wheat varieties to FHB with the exception that all durum varieties are very susceptible. The level of FHB infection is heavily related to climatic conditions during flowering with minor differences in flowering time potentially giving dramatic differences in the level of infection.
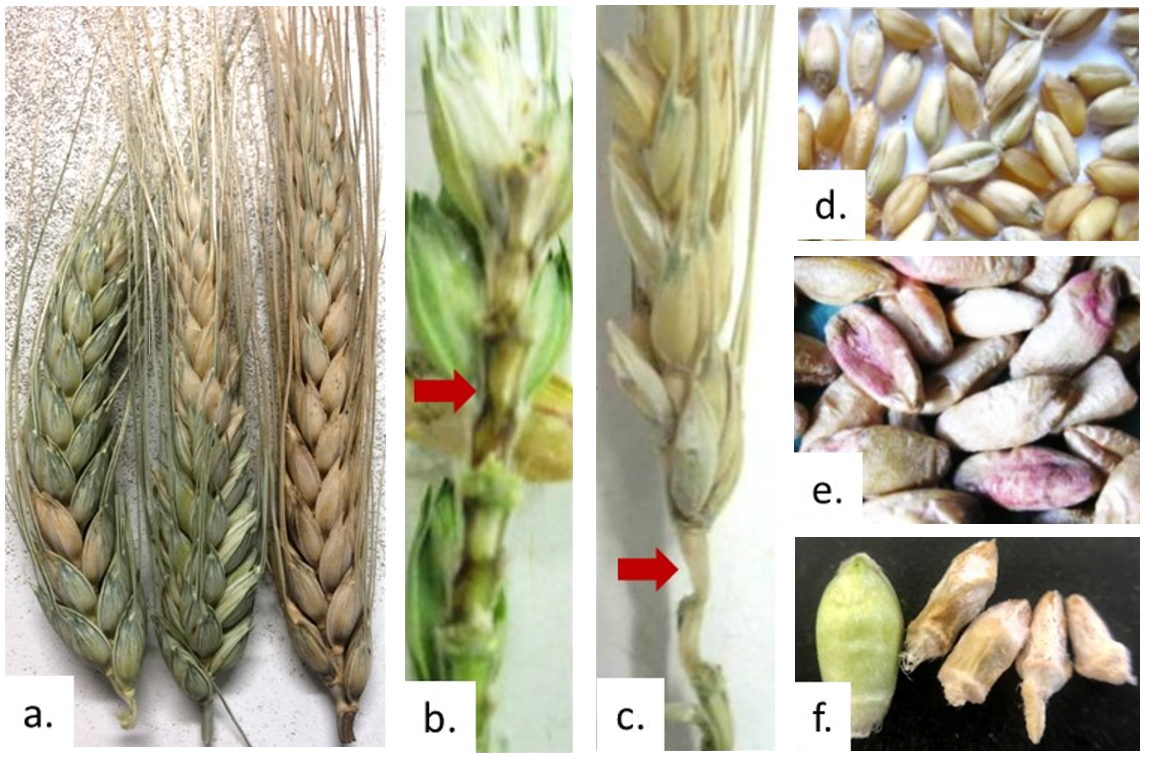
Figure 1. Correct diagnosis of Fusarium head blight and white grain disorder.
a. FHB and WGD both cause partial bleaching or total bleaching of heads.
b. If FHB is present, then the stem in the head (rachis) will be brown at the point where bleached spikelets attached.
c. If WGD the rachis will be white where bleached spikelets attach.
d. Both FHB and WGD cause production of white grains.
e. If there is any pink coloration of grain, then this is diagnostic of FHB.
f. Depending on infection timing, infected grains are often pinched and lighter and hence the majority blow out the back of the header at harvest. Try increasing header fan speed in infected paddocks or paddocks to be retained for seed. However, this only works if infection was early in the flowering/grain fill period and resulted in pinched grains.
Why is species identification important?
Knowing the Fusarium species of FHB or whether it is WGD is important to determining likely inoculum source and management going forward. FHB caused by Fusarium graminearum (Fg) likely produces larger quantities of more toxic forms of mycotoxins (15ADON and nivalenol) based on Australian studies in 2010 and 2016. FHB caused by Fusarium pseudograminearum (Fp) likely produces lower quantities of a less toxic form of DON (3ADON). WGD caused by Eutiarosporella spp. (Eut) produces no known mycotoxins (Simpfendorfer et al. 2017).
Species identification using quantitative PCR of 218 head or grain samples from 2022 submitted from across NSW and southern Qld shows FHB caused by Fp as the dominant issue (60% Fp only) followed by FHB caused by Fg (5% Fg only) and WGD caused by Eut (5% Eut only). Mixed infections occurred within some cereal crops with Fp + Eut (19%) most common followed by Fp + Fg (11%), Fg + Eut (1%) and Fp + Fg + Eut (1%).
Caution feeding infected grain to livestock
Take care when feeding Fusarium infected grain to stock. There are no specific Australian stock feed guidelines for mycotoxins. The US Food and Drug Administration (FDA) have guidelines that state: for the main DON toxin, advisory levels for food products consumed by humans is 1 part per million (ppm); 10 ppm for ruminating beef and feedlot cattle older than 4 months (cannot exceed 50% of diet); 10 ppm for poultry (cannot exceed 50% of diet); 5 ppm for swine (cannot exceed 20% of diet); and 5 ppm for all other animals (cannot exceed 40% of diet)(US Food and Drug Administration 2010).
Are there issues if retaining grain for sowing?
Absolutely because Fusarium and Eutiarosporella infection both reduce germination and can cause early seedling death (blight). Fusarium infected grain can also introduce Fusarium crown rot into clean paddocks through seed infection. The level of infection can be higher than the visual level of white or pink grains, as later infections may not discolour the seed. Up to 70% Fusarium grain infection has been measured in one durum wheat crop sent in from near Hillston from the 2022 harvest. So far, grain infection levels are generally much higher in seed retained from durum crops compared with bread wheat, highlighting their increased susceptibility to FHB. However, 20-30% Fusarium grain infection has been measured in a number of bread wheat seed sources retained from the 2022 harvest.
There are registered fungicide seed treatments to reduce the extent of seedling blight when sowing Fusarium infected grain. However, once infection levels get over 5% its best to try and find a cleaner seed source, if possible, as higher infection levels are also often linked to poor seedling vigour. Grading out lighter seed prior to sowing can also help as this will remove obvious severely infected grains.
Fungicide seed treatments will not eliminate Fusarium crown rot infections associated with sowing into infected cereal stubble or grass weed residues in a paddock and have no effect on FHB later in the season.
Could I have sprayed to stop it?
The only registered fungicide product to control FHB is Prosaro® 420 SC, which needs to be applied to protect the flowers at heading, follow label instructions. Research has shown that spraying at flowering (GS61) was more effective and had more yield benefit than spraying seven days before flowering. The anthers (flowers) are the primary infection site for FHB, so spraying before flowering provides reduced protection of these plant structures.
Overseas research has demonstrated the importance of spray coverage in FHB control, with twin nozzles (forward and backward facing) angled to cover both sides of a wheat head and high volumes of water (≥100 L/ha) being critical to efficacy. However, at best this still provides ~80% control. Aerial application gives poor coverage of heads and at best provides ~40 to 50% control. Some agronomists who used this application method in 2022 are questioning if the efficacy is even this high following their experience.
Prosaro® 420 SC is only usually applied to durum wheat (very susceptible to FHB) in parts of northern NSW which have dealt with FHB since 1999. Application to bread wheats has never previously been deemed economical but infection levels in many bread wheat crops in 2022 have challenged this thinking. Note, in north America strobilurin fungicides are not recommended from booting (GS45) onwards in paddocks with FHB risk as this can increase mycotoxin accumulation in infected grain (Chilvers et al. 2016).
Harvest considerations
Harvest order or separation – Infection levels vary from paddock to paddock. Ideally, each paddock’s grain should be binned separately to optimise market opportunities. Based on assessments of FHB just after flowering, the harvesting of heavily infected paddocks or sections of paddocks may be abandoned or sold directly for feed. Alternatively, more heavily infected sections of a paddock may be harvested separately from the rest of the crop. Levels of FHB may also alter the priority in which individual paddocks are harvested. FHB damaged grain must also be stored properly to prevent further disease development. Grain infected with FHB with a moisture content greater than 13% should be dried to stop further mould and mycotoxin development.
Header set-up – Adjust header openings and wind so that shrivelled, light weight, infected grain is removed along with the stubble. This technique will also reduce the level of mycotoxins, if present, in the harvested grain and is one reason why high concentrations of toxins usually do not end up in harvested grain and eventually the milled product. However, this will not remove all infected seed, since some FHB infection occurs late in development of the grain, and these infected seeds may still be plump. This technique is also only an option when the rest of the grain is of good quality. In paddocks severely affected by leaf diseases (e.g., yellow spot), which are also favoured by warm moist conditions, separating shrivelled grain caused by leaf disease and FHB is not possible during the harvesting process.
Mixing with uninfected sections or paddocks – Sections of a paddock with low levels of FHB infection could be harvested separately and blended with uninfected grain from the rest of the crop to reduce infected seed below receival limits. Equally, grain from an uninfected paddock can be mixed with seed from an infected paddock if the combined grain remains below quality limits set at the receival point. This practice may be too risky if trying to mix grain harvested from a paddock heavily infected with FHB. A combination of gravity grading followed by blending with uninfected grain may be required under moderate disease levels.
Gravity grading – This technique can be used to remove a large proportion of the light weight, pinched, chalky white and/or pink FHB infected grain before delivery to the silo to hopefully limit downgrading or allow delivery. This technique may also reduce the level of Fusarium grain infection if retaining seed for sowing. This technique is probably not viable under severe infection from FHB when most of the grain is diseased.
Human safety precautions – FHB damaged crops can be harvested and handled safely, provided normal precautions are taken to avoid exposure to grain dust. Grain dust is a hazardous substance, regardless of whether the Fusarium fungus is present. Various fungi and moulds in the dust can cause allergic reactions and lung irritation, and prolonged exposure can lead to serious breathing problems. Growers should take all the same precautions they would if handling mouldy grain. These precautions include using masks, goggles and protective clothing.
Summary
The 2022 season with prolonged high humidity (>80%) during flowering and grain fill was extremely conducive to FHB and WGD infection and development. Extended cool conditions which prolonged the flowering period were also likely a big factor in the increased prevalence of FHB and WGD this season.
If FHB is the result of basal infection of Fusarium crown rot, then the underlying issue needs to be rectified through an integrated disease management plan including crop rotation. Determining the cause of FHB or WGD is important when providing appropriate future management advice. In the majority of situations tested so far it was the FCR fungus (Fp) reminding us that it does not go away in wet years. If grain fill conditions had been hot and dry what would the level of whitehead expression and yield loss from FCR been in your crop?
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers and their advisers through their support of the GRDC. The author also acknowledges the ongoing support for northern pathology capacity by NSW DPI.
Further information
Chilvers M, Nagelkirk M, Tenuta A, Smith D, Wise K and Paul P (2016) Managing wheat leaf diseases and Fusarium head blight (head scab), Michigan Stat University, MSU Extension.
Simpfendorfer S, Giblot-Ducray D, Harley D and McKay A (2017) Where did the low levels of Fusarium head blight come from in 2016 and what does it mean, GRDC Update Paper.
US Food and Drug Administration (2010) Guidance for industry and FDA: Advisory levels of deoxynivalenol (DON) in finished wheat products for human consumption and grains and grain by-products used for animal feed
Contact details
Steven Simpfendorfer
NSW DPI, 4 Marsden Park Rd, Tamworth, NSW 2340
Ph: 0439 581 672
Email: steven.simpfendorfer@dpi.nsw.gov.au
Twitter: @s_simpfendorfer or @NSWDPI_AGRONOMY
Brad Baxter
NSW DPI, Pine Gully Rd, Wagga Wagga, NSW 2650
Ph: 0428 294 121
Email: brad.baxter@dpi.nsw.gov.au
Twitter: @BradBaxter1985 or @NSWDPI_AGRONOMY
Testing of grain infection levels
Send 200-250 g seed in plastic double zip lock bags with variety and location to Steven Simpfendorfer at Tamworth laboratories (above). No charge as funded by GRDC project.
Pre-sowing paddock FCR/FHB risk
PREDICTA®B soil/stubble testing available through SARDI.
Or alternatively contact Steven Simpfendorfer or your Local Land Services office about stubble testing.
Podcasts
NSW DPI podcasts: are now on popular streaming platforms, such as Apple and Spotify. Just search for NSW DPI Agronomy. Alternatively, you can subscribe and receive NSW DPI podcasts on Soundcloud.
Date published: February 2023
® Registered trademark
GRDC Project Code: DPI2207-002RTX, DPI2207-004RTX,