Insecticidal control of green peach aphid and turnip yellows virus – resistance threats, limitations and future alternatives
Insecticidal control of green peach aphid and turnip yellows virus – resistance threats, limitations and future alternatives
Author: Ben Congdon, Lisa Kirkland and Paul Umina | Date: 27 Feb 2023
Key messages
- ‘Low-level’ metabolic resistance in green peach aphid (GPA) reduced the effectiveness of neonicotinoid-based seed treatments on canola under semi-field conditions.
- In two field trials, a pre-emptive foliar application of insecticide in large plots of canola limited GPA populations but was generally insufficient to significantly suppress turnip yellows virus (TuYV) spread.
- Even small populations of aphids can cause significant TuYV spread – virus management focussed on controlling the aphid vector must be highly effective to have an impact on TuYV infection levels.
- Partial resistance to TuYV found in ATR Stingray was highly effective at suppressing TuYV spread, and suppression by resistance was not enhanced by insecticide.
Introduction and Aims
Insecticidal pest control in food crops worldwide is becoming increasingly challenging with widespread insecticide resistance in target pests and legislation restricting insecticide use in certain jurisdictions. Most notably, the European Union recently banned the use of neonicotinoids in all field crops, which has caused major pest control issues in crops such as canola and sugar beet (Dewar and Qi 2021). The impact of trends restricting the use of insecticides is being exacerbated by the decreased rate at which new modes of action are registered (S&P Global Commodity Insights 2018). In Australian grain crops, insecticidal control has been fundamental to the management of many important pests.
The green peach aphid (Myzus persicae – GPA) imparts its greatest damage to canola crops by vectoring turnip yellows virus (TuYV). Since their introduction into the Australian grains industry, neonicotinoid-based seed treatments have been widely used to manage this threat, in part because they can be applied prophylactically at scale and provide systemic protection of young plants most vulnerable to viral infection (Jones et al 2007). Concerningly, GPA has evolved multiple mechanisms that confer resistance to neonicotinoids including high level resistance (>1,000-fold under laboratory conditions) conferred by the R81T mutation, which has not been found in Australia (McGrane et al 2021). A more common resistance mechanism conferred by amplification of the cytochrome P450 gene, CYP6CY3, is widespread in Australian GPA populations (de Little et al 2017). While CYP6CY3 amplification has been associated with low-level resistance to several neonicotinoids in laboratory-based acute toxicity bioassays (~5 to ~25-fold), its impact was thought to be of little practical significance. However, the impact of CYP6CY3 amplification on the field effectiveness of neonicotinoids when applied at recommended field rates warrants investigation.
For control of barley yellow dwarf virus (BYDV) in cereals, pre-emptive synthetic pyrethroid applications have been shown to suppress virus spread (McKirdy and Jones 1996). This is in part due to the anti-feed attributes of synthetic pyrethroids. BYDV is a similar virus to TuYV, but very little data is available demonstrating the effectiveness of foliar insecticide applications for suppressing TuYV spread in canola. However, resistance to synthetic pyrethroids, and several other common foliar actives, is widespread in Australian GPA populations (Umina et al 2014). Currently, there are several chemical active ingredients registered against GPA in canola, including sulfoxaflor (Transform®), flonicamid (MainMan®) and afidopyropen (Versys®). Each of these are reported to be systemically active with residual efficacy and anti-feed activity and therefore have potential as virus control tools. Nonetheless, insecticide control of TuYV may be challenging as GPA transmits TuYV with extremely high efficiency; just one or two individuals are enough to infect a susceptible plant. Therefore, the insecticide needs to be highly effective at suppressing GPA colonisation to significantly suppress TuYV spread. Furthermore, achieving coverage of insecticide on each plant sufficient to ensure effective control may be limited by canola canopy architecture coupled with GPA’s tendency to colonise lower leaves.
In the EU, partly in response to bans on neonicotinoids, researchers and breeding companies have collaborated to produce commercially available virus resistant varieties (e.g., Aspire by Limagrain), which are proving to be effective under high disease pressure. Furthermore, researchers have continued to identify several additional and promising sources of resistance (Greer et al 2021). In contrast, in Australia, there are almost no commercially available canola varieties with TuYV resistance. One possible exception is ATR Stingray (Nuseed Australia), which has shown promising partial resistance to TuYV infection under glasshouse and field conditions (van Leur et al 2015). Resistant varieties may provide a viable solution to help reduce the burden on insecticidal control, but they need to be shown to be effective under Australian conditions to warrant investment.
This paper describes a series of experiments designed to investigate (i) the impact of CYP6CY3 amplification on the ability of GPA to survive neonicotinoid exposure under semi-field conditions, and (ii) the effectiveness of a pre-emptive application of insecticide, a neonicotinoid seed treatment, and partial plant host resistance to TuYV in suppressing TuYV spread under field trial conditions.
Materials and methods
Semi-field microcosm trial
A large-scale trial in a shade house was conducted to determine the impact of CYP6CY3 copy number on the efficacy conferred by two insecticide seed treatments when applied to canola. The GPA clones used were Kyabram98 (1x copy) collected in Victoria in 2002 and laboratory cultured since, EastNaernup209 (3x copies) collected from Western Australia in 2018 and Osborne171 (6x copies) collected from Queensland in 2020. The seed used was of canola variety Hyola TT (Pacific Seeds) and was divided into three equal quantities and treated at label rate with either Cruiser® Opti (Syngenta Australia) or Gaucho® (Bayer CropScience) or left ‘untreated’.
Seeds from each treatment (untreated, Cruiser® Opti, and Gaucho®) were sown singly into square propagation pots containing a soil mix of 2 parts loam:1 part sand. Seeds were sown fortnightly to achieve canola plants at five different growth stages, aged 2, 4, 6, 8 and 10 weeks after emergence (WAE) at the time of aphid introduction. On each sowing date, 24 pots for each treatment (i.e., 72 plants in total) were established. Plants were grown in ventilated exclusion cages in a randomised fashion within the shade house. Before aphid introductions, two replicate plants from the same treatment and growth stage were placed together within a sealed plastic microcosm tub (18cm L x 12cm W x 52cm H) ventilated with mesh windows. For each chemical treatment and WAE combination, there were four replicate tubs with two plants in each (i.e., 360 plants and 180 tubs in total). Once plants were at the desired growth stage, 100 individuals from each of the three GPA clones were introduced into designated tubs, and the tubs sealed. Tubs were randomised within the shade house and rotated regularly. The total number of aphids within each tub was then directly counted 14 days after introduction (DAI).
Field trials
Northam 2020
In 2020, a field trial was established at Northam to examine the effectiveness of a pre-emptive application of insecticide to control GPA and TuYV in the canola variety ATR Bonito (Nuseed Australia). Field plots were sprayed with either alpha-cypermethrin at 12.5gai/ha, sulfoxaflor at 24gai/ha or 48gai/ha, or flonicamid at 25gai/ha or 50gai/ha or left untreated. The alpha-cypermethrin is the labelled rate for the prevention of BYDV transmission in Barley. . There were 4 replicate plots per treatment. Each plot was 8 x 10m, consisting of 5 plot seeder runs. At the 3-5 leaf growth stage, the plots were sprayed with their respective insecticide treatments (or left untreated). Three days later, TuYV-infected canola plants that were infested with GPA were deployed equidistantly throughout the trial so that each plot had an infector plant on each of its corners. The total number of aphids on four plants down the centre row of each seeder run of each plot (20 plants in total) were then counted at 2, 4 and 6 weeks after aphid introduction (WAAI). In the same manner, the youngest fully formed leaf from 20 plants were taken from each plot at the same time points and tested for the presence of TuYV infection by enzyme-linked immunosorbent assay (ELISA). Due to severe drought conditions throughout flowering and podding, the plants produced minimal seed and so yield measurements were not taken.
Muresk 2022
In 2022, a second field trial was established at Muresk to examine the effectiveness of a pre-emptive application of insecticide and a neonicotinoid-based seed treatment (Cruiser® Opti) to control GPA and TuYV in a susceptible (ATR Bonito) and partially resistant (ATR Stingray) variety. Plots were sown with seed that was either treated or untreated with the neonicotinoid-based seed treatment. At the 3-5 leaf growth stage, Plots were later sprayed with either sulfoxaflor at 48gai/ha, flonicamid at 50gai/ha, or afidopyropen at 5gai/ha or left untreated. The trial followed the same layout and aphid introduction method as the Northam trial, except there were 6 replicate plots per treatment. Aphid counts and sample collection for virus testing occurred in the same manner as the Northam trial, except that virus testing was also undertaken at 8-WAAI. Seed yield measurements for each plot were taken from two plot-harvester sub-samples per plot.
Results and Discussion
Semi-field microcosm trial
As expected, both Gaucho® and Cruiser® Opti seed treatments provided full control of the susceptible clone (Kyabram98), including when aphids were introduced to canola seedlings 10-WAE (Figure 1). Conversely however, these seed treatments offered relatively little control at any growth stage tested of Osborne171 aphids, which had the highest CYP6CY3 copy number. In addition, both seed treatments conferred only partial control of EastNaernup209. These results suggest that neonicotinoid resistance mediated through an increased copy number of CYP6CY3 has greater practical significance for GPA control than previously understood and implies growers may encounter difficulties when attempting to control GPA with neonicotinoid seed treatments in Australia. This has particular importance for the management of plant viruses transmitted by aphids. Although partial control of GPA could be enough to prevent yield losses because of direct feeding damage, it is unlikely to be sufficient to prevent virus transmission.
For more details about this work see Kirkland et al (2023).
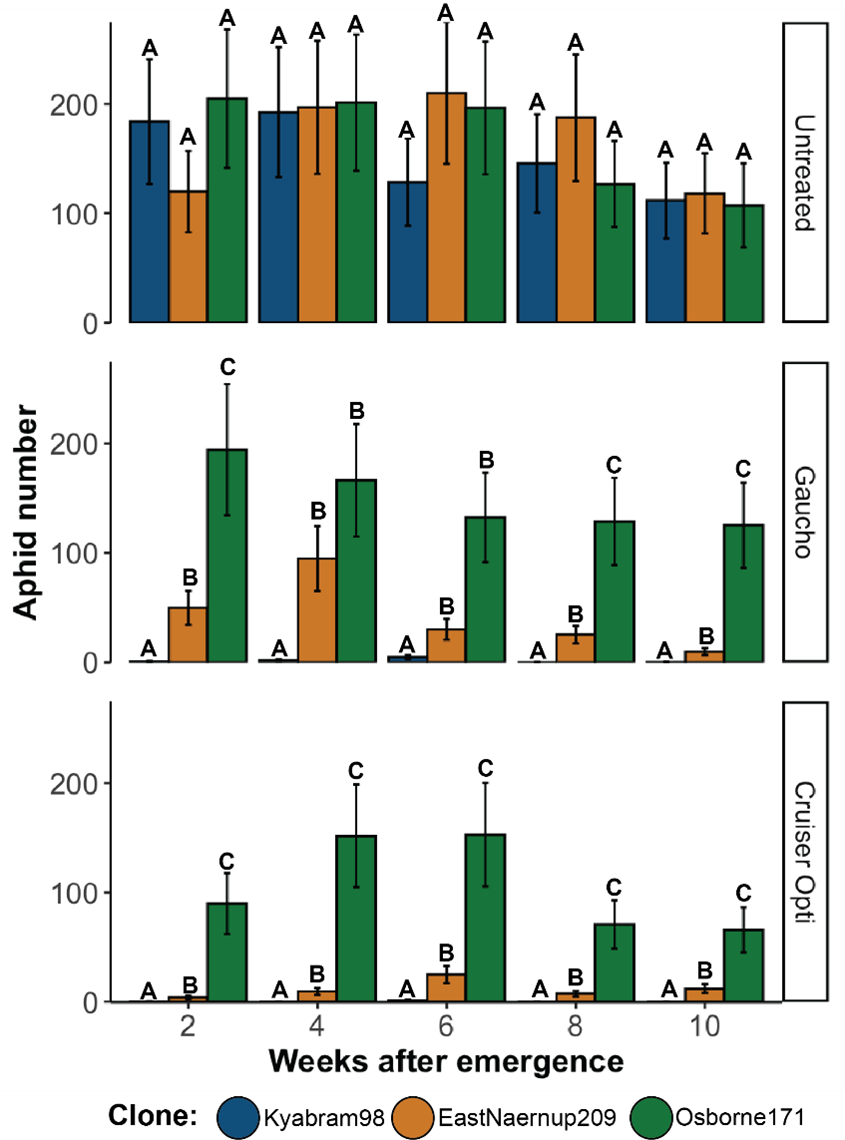
Figure 1. Average numbers (± standard error) of GPA 14 days after introduction to canola plants grown from untreated, Gaucho® (imidacloprid) or Cruiser® Opti (thiamethoxam and lambda-cyhalothrin) seed. Capital letters above error bars denote significant differences between clones within each WAE
Field trials
Northam 2020
Compared with the untreated plots, GPA populations were suppressed in plots treated with sulfoxaflor and flonicamid at 2, 4 and 6-WAAI, but not with the alpha-cypermethrin application (Figure 2a). The lack of control in the alpha-cypermethrin treatment was unsurprising as almost all GPA clones in Australia possess a pyrethroid resistance allele. At 6-WAAI, aphid numbers across the trial decreased slightly from 4 to 6 weeks after introduction, likely due to predation by wasps.
There was a ~4-week latency period before widespread TuYV infection was detected in the Northam trial, with incidences spiking dramatically at 6-WAAI for all treatments (Figure 2b). The only spray treatment to have significantly lower infection levels compared with the untreated control (93% infection) at 6-WAAI was sulfoxaflor at the 48gai/ha rate, but even then, the infection rate was >50%. Pre-emptive application of both sulfoxaflor and flonicamid significantly suppressed aphids indicating residual control during the early stages of aphid colonisation. However, these applications were mostly insufficient at effectively suppressing virus infection.
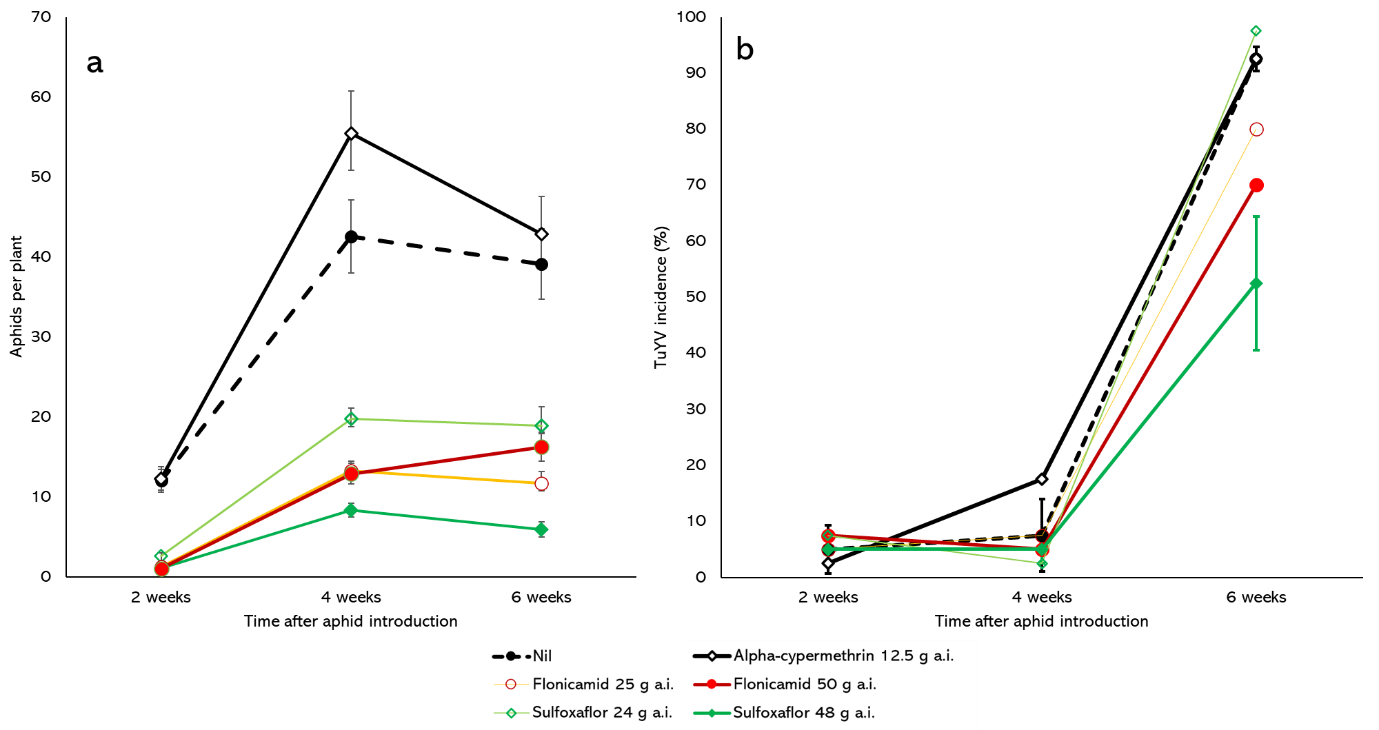
Figure 2. Number of aphids per plant (a) and % plants infected with TuYV (b) at different time points after viruliferous aphid introduction in plots of canola cv. ATR Bonito sown at Northam in 2020
Muresk 2022
Compared with the untreated plots, GPA were suppressed in plots treated with all insecticide treatments at 2 and 4 WAAI but not at 6-WAAI (Figure 3a). Overall, aphid numbers were 5 to 6-fold lower and the % of plants colonised was lower than at the Northam trial, likely due to cooler and wetter conditions experienced during the data collection period. As a consequence of this slower aphid movement and smaller population size, TuYV infection levels were also lower. On ATR Bonito, although there were slightly lower TuYV infection levels in the insecticide treated plots than the untreated plots, these differences were not statistically significant (Figure 3b). Interestingly, aphid numbers and virus infection levels did not significantly differ between sulfoxaflor treated plots sown with neonicotinoid treated seed versus untreated seed.
When comparing canola varieties with differing levels of resistance, in all insecticide treatments and at all WAAI, the partially resistant ATR Stingray had significantly lower levels of TuYV infection (<5%) than ATR Bonito (35%) (Figure 4).
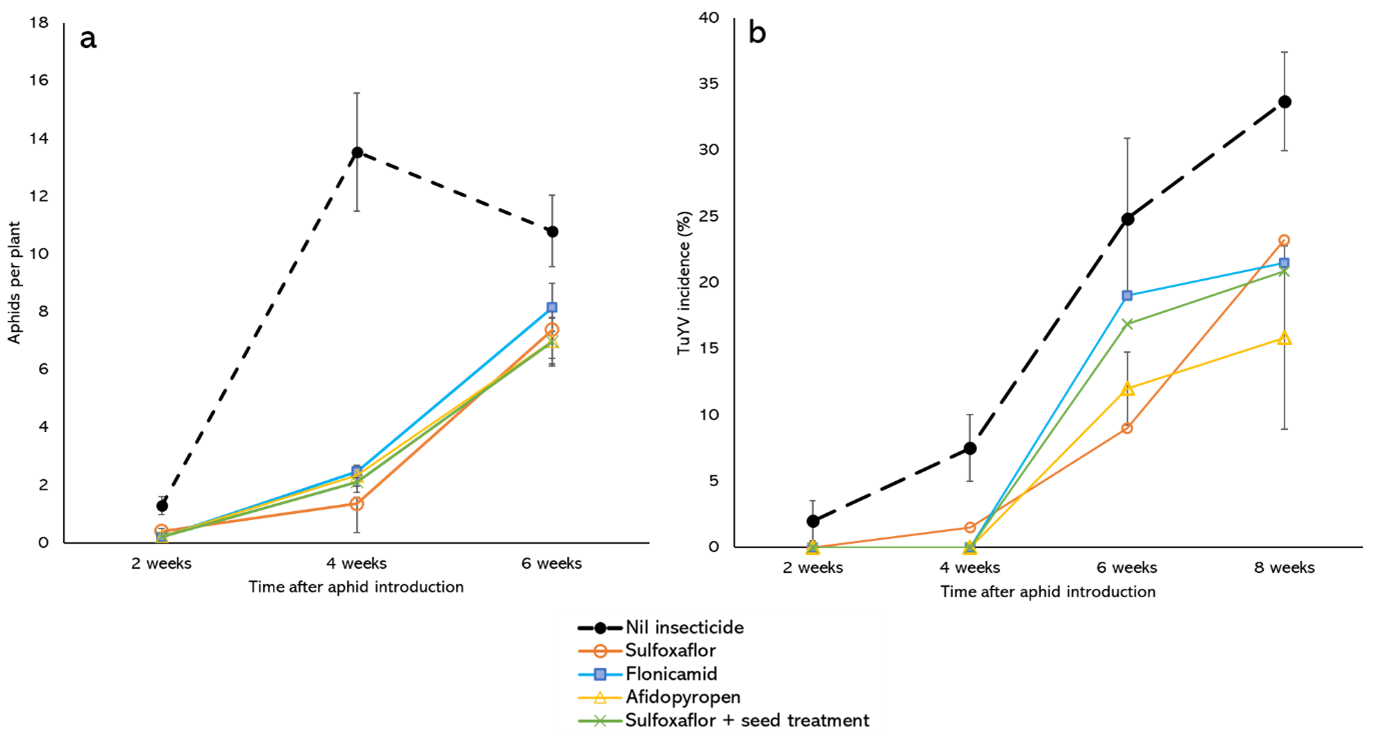
Figure 3. Number of aphids per plant (a) and % plants infected with TuYV (b) at different time points after viruliferous aphid introduction in plots of canola cv. ATR Bonito sown at Muresk in 2022
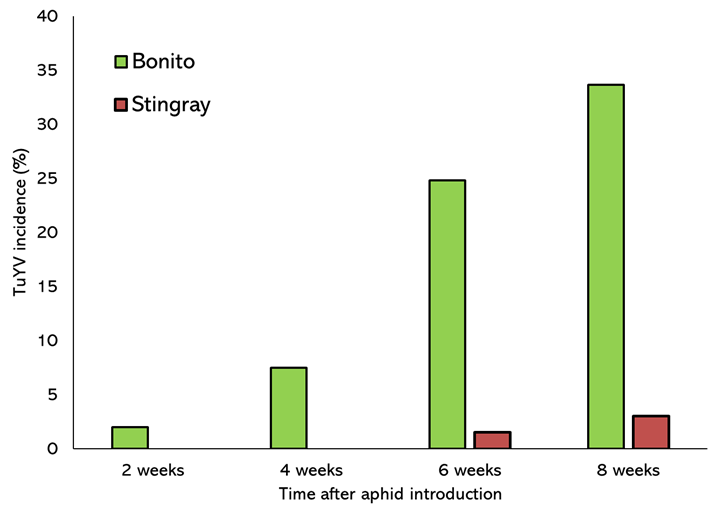
Figure 4. Mean % plants infected with TuYV at different time points after viruliferous aphid introduction across all plots of ATR Bonito and ATR Stingray sown at Muresk in 2022
There was a significant 15% reduction in yield in plots with moderate levels (>20%) of TuYV infection (Figure 5a). ATR Bonito yielded significantly less (7%) than ATR Stingray (Figure 5b), despite consistently yielding higher than ATR Stingray at moderate-high rainfall sites in the past five seasons of NVT trials in Western Australia (nvtonline.com.au).
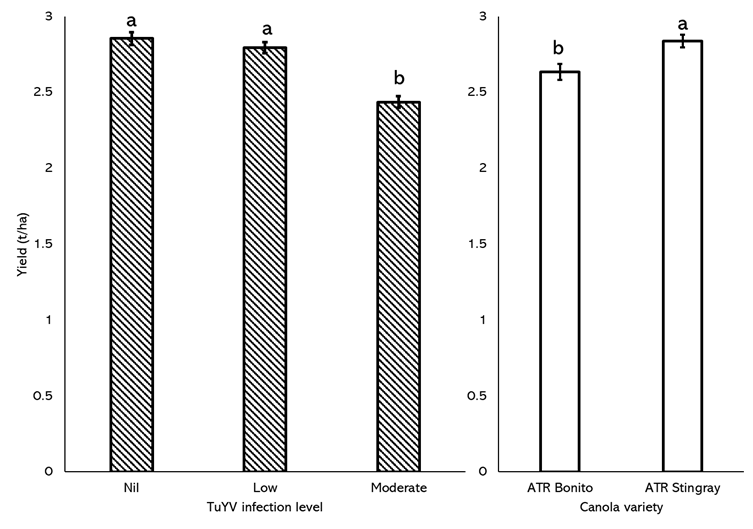
Figure 5. Mean seed yield (t/ha) comparison between plots with nil (0% infection), low (0 to 20%) and moderate (>20%) levels TuYV infection (a) and between plots of ATR Bonito and ATR Stingray (b) sown at Muresk in 2022. Letters above error bars denote significant differences between treatments
Similar to the Northam trial, a single pre-emptive application of insecticide suppressed GPA colonisation at the Muresk trial but was insufficient to significantly suppress virus infection. In contrast to insecticides, partial resistance as observed in ATR Stingray was far more effective in reducing TuYV infection. Furthermore, even with TuYV spread occurring quite late in the data collection period and only reaching moderate levels, economically significant seed yield losses of 420kg/ha (15%) occurred.
Conclusions
The continued evolution of insecticide resistance in GPA coupled with uncertainties around legislation of key insecticides, particularly neonicotinoids, indicates new control options for GPA and TuYV in canola are required.
The semi-field trial examining the impact of insecticide resistance in GPA on the efficacy of neonicotinoid-based seed treatments was surprising and may prove to be a concern for growers. Sub-optimal aphid control at early plant growth stages (as seen in this study in the resistant clones) means virus infections can potentially occur quite early in crop development, which for many plant viruses, including TuYV, will result in greater yield losses than if the failure occurred at later plant growth stages (Congdon et al 2020). Furthermore, it is likely that widespread neonicotinoid insecticide use in Australia is a major selective force driving the observed geographic expansion of this resistance since its initial detection. Trials that directly test the efficacy of neonicotinoid seed treatments against virus transmission by GPA clones possessing different copy numbers of CYP6CY3 under true field conditions are warranted as a next step.
The field trial results presented here demonstrate that TuYV resistance in canola has the potential to be an effective and less management-intensive way to reduce the on-farm economic and environmental burden of insecticides. The next research step in developing commercially available resistant varieties is to test known sources of resistance from Australia and overseas against a broader genetic spectrum of TuYV strains. In addition, further research is required to examine whether foliar insecticides are indeed useful for controlling TuYV. The differences in disease pressure in natural conditions at the paddock scale compared with the spatial limits on plot sizes and contrived disease pressure of a field trial could be important to determining the real-world effectiveness of foliar insecticides. Reducing the speed at which GPA colonises the crop is likely to have a greater impact on virus spread at the paddock scale. Aphid incursion may begin in one corner of the paddock and the insecticide may significantly reduce the speed at which aphid populations establish across a paddock that is 100-1000 times larger than an experimental plot. In addition, an investigation into alternative spray regimes in managing TuYV (e.g., spraying after initial GPA colonisation or doing a second spray application with a different mode-of-action) would be worthwhile.
Acknowledgements
We thank Nuseed, Corteva, UPL, Syngenta Australia, Nufarm Australia for providing seed and chemical, and DPIRD Northam Research Services Unit and Living Farm for field trial sowing, maintenance and harvest. We also thank Evatt Chirgwin, Samantha Ward, Anthony van Rooyen, Marielle Babineau, Leo McGrane, J. Baulch, A. Balfour-Cunningham, F. Rehman, U. Poudel, U. Khanal for technical and laboratory support. The semi-field trial was funded by DPIRD Boosting Grains project 2019SP02 and GRDC project CES2001-001RTX, and the field trials by DPIRD Boosting Grains project 2019SP02.
References
Congdon, B. S., J. R. Baulch and B. A. Coutts (2020). "Impact of Turnip yellows virus infection on seed yield of an open-pollinated and hybrid canola cultivar when inoculated at different growth stages." Virus Research 277: 197847
de Little, S. C., O. Edwards, A. R. van Rooyen, A. Weeks and P. A. Umina (2017). "Discovery of metabolic resistance to neonicotinoids in green peach aphids (Myzus persicae) in Australia." Pest Management Science 73(8): 1611-1617
Dewar, A. and A. Qi (2021). "The Virus Yellows Epidemic in Sugar Beet in the UK in 2020 and the Adverse Effect of the EU Ban on Neonicotinoids on Sugar Beet Production." Outlooks on Pest Management 32: 53-59
Greer, S., D. Hackenberg, V. Gegas, G. Mitrousia, D. Edwards, J. Batley, G. Teakle, G. Barker and J. Walsh (2021). "Quantitative Trait Locus Mapping of Resistance to Turnip Yellows Virus in Brassica rapa and Brassica oleracea and Introgression of These Resistances by Resynthesis Into Allotetraploid Plants for Deployment in Brassica napus." Frontiers in Plant Science 12: 781385.
Jones, R. A. C., B. A. Coutts and J. R. Hawkes (2007). "Yield-limiting potential of Beet western yellows virus in Brassica napus." Australian Journal of Agricultural Research 58(8): 788-801
Kirkland, L. S., E. Chirgwin, S. E. Ward, B. S. Congdon, A. van Rooyen and P. A. Umina (2023). "P450-mediated resistance in Myzus persicae (Sulzer) (Hemiptera: Aphididae) reduces the efficacy of neonicotinoid seed treatments in Brassica napus." Pest Management Science
McGrane, L., F. Noakes, P. A. Umina, J. Maino, J. Lye and A. Arthur. (2021). "Pesticide resistance in Australian grain regions – lessons to be learnt." GRDC Research Updates Retrieved 6/9/21, from https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2021/02/pesticide-resistance-in-australian-grain-regions-lessons-to-be-learnt
McKirdy, S. J. and R. A. C. Jones (1996). "Use of imidacloprid and newer generation synthetic pyrethroids to control the spread of barley yellow dwarf luteovirus in cereals." Plant Disease 80(8): 895-901
S&P Global Commodity Insights (2018). Evolution of the crop protection industry since 1960.
Umina, P. A., O. Edwards, P. Carson, A. Van Rooyen and A. Anderson (2014). "High levels of resistance to carbamate and pyrethroid chemicals widespread in Australian Myzus persicae (Hemiptera: Aphididae) populations." J Econ Entomol 107(4): 1626-1638
van Leur, J., K. Lindbeck, M. Aftab, A. Freeman and D. McCaffery (2015). "Virus development in canola crops during 2014 in New South Wales and implications for the oilseed and pulse industry." GRDC Update Papers.
Contact details
Ben Congdon
Department of Primary Industries and Regional Development
Ph: (08) 9368 3499
E: Benjamin.Congdon@dpird.wa.gov.au
