Pesticide resistance in Australian grain regions – lessons to be learnt
Pesticide resistance in Australian grain regions – lessons to be learnt
Author: Leo McGrane and Francesca Noakes (cesar Australia), Paul A. Umina (cesar Australia and University of Melbourne), James Maino, Jessica Lye and Aston Arthur (cesar Australia). | Date: 09 Feb 2021
Take home messages
- An over-reliance on broad-spectrum pesticides, and a limited number of registered chemicals available, creates strong selection pressure for resistance in grain pests.
- The redlegged earth mite (Halotydeus destructor; RLEM) and the green peach aphid (Myzus persicae; GPA) are two important grain pests that have evolved resistance to multiple pesticide groups in Australia. Surveillance demonstrates that resistance is expanding, highlighting the need for a reassessment of management approaches.
- Taking an integrated approach to pest management limits the need for prophylactic pesticide applications by utilising biological and cultural control options, thereby reducing the likelihood of further pesticide resistance evolution.
- Adoption of resistance management strategies (RMSs), and principles described in recently developed best management practice guides (BMPGs), will reduce selection for resistance and prolong the long-term viability of chemical control.
Background
Within the Australian grains industry, increasing cases of pesticide resistance and diminishing chemical options available to growers is necessitating a renewed focus on pest management practices. Several important invertebrate pests of grain crops have evolved pesticide resistance in Australia. These include Helicoverpa armigera (cotton bollworm), Plutella xylostella (diamondback moth), Myzus persicae (green peach aphid, GPA) and Halotydeus destructor (redlegged earth mite, RLEM) (for overview, see Umina et al. 2019). Most of these species have evolved resistance to multiple chemical classes both in Australia and overseas. Recent forecasting, described in McDonald et al. (2019), has further identified several important grains pests at high risk of evolving resistance in the future (including Bryobia mite and Lucerne flea) which currently have no known resistances to pesticides in Australia.
This paper focuses on the evolution of pesticide resistance in RLEM and GPA in Australia, describing current research and reporting on recent resistance detection work. Also presented is the research examining the responses of Bryobia mite species to different pesticides. Finally, integrated approaches to pest management that could limit prophylactic pesticide applications are discussed.
Pesticide trends
A key driver of increased pesticide resistance in Australian grain regions is the over-reliance on a limited number of chemical Mode of Action groups; Group 1 (carbamates 1A and organophosphates), Group 3A (pyrethroids) and Group 4A (neonicotinoids) (Umina et al. 2019). This over-reliance is underpinned by a number of factors. The large, mechanised nature of Australian grain farms and a zero-tolerance stance for live invertebrates in grain exports has led to the necessity of controlling pest outbreaks on farms. Furthermore, the climatic variability of many Australian grain growing regions makes it difficult to judge the risk of pest outbreaks, often leading to the application of ‘insurance sprays’.
In recent years, costs associated with developing a new pesticide have increased exponentially, while the rate of successful development of new pesticides has decreased (Figure 1). A lack of new chemical actives being registered to the Australia market has intensified the use of existing options for RLEM and GPA.
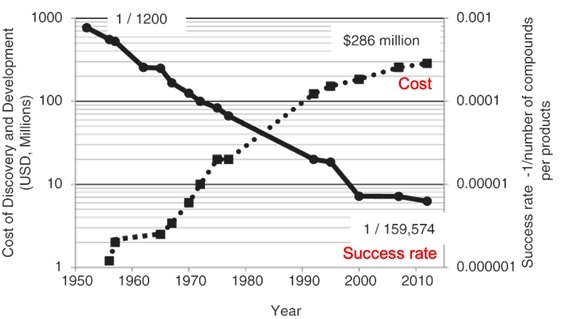
Figure 1. Log scale of pesticide development cost (dotted line) vs pesticide development success rate (solid line) (Image credit: Sparks 2017).
Resistance in the redlegged earth mite
The RLEM is one of the most destructive and economically important pests of winter grain crops and pastures in southern Australia. It is particularly damaging at the establishment phase of plants in autumn (Ridsdill-Smith et al. 2008; Umina and Hoffmann 2004). Resistance to synthetic pyrethroids in RLEM was first detected in Western Australia (WA) in 2006, followed by the detection of organophosphate resistance in 2014 (Maino et al. 2018; Umina 2007; Umina et al. 2012; Umina et al. 2017). The continued detection of resistant populations in new regions, such as South Australia (SA) and Victoria (Vic), has resulted in a reassessment of management approaches for this pest.
Control of RLEM currently relies heavily on the application of pesticides through contact sprays or seed treatments (Ridsdill-Smith et al. 2008; Umina et al. 2017). There are currently five chemical Mode of Action groups registered for control of RLEM in Australia; organophosphates (Group 1B), fiproles (Group 2B), synthetic pyrethroids (Group 3A), neonicotinoids (Group 4A) and diafenthiuron (Group 12A) (APVMA, 2020). Of these, growers rely heavily on three; organophosphates, synthetic pyrethroids and neonicotinoids (Umina et al. 2019) (Figure 2).
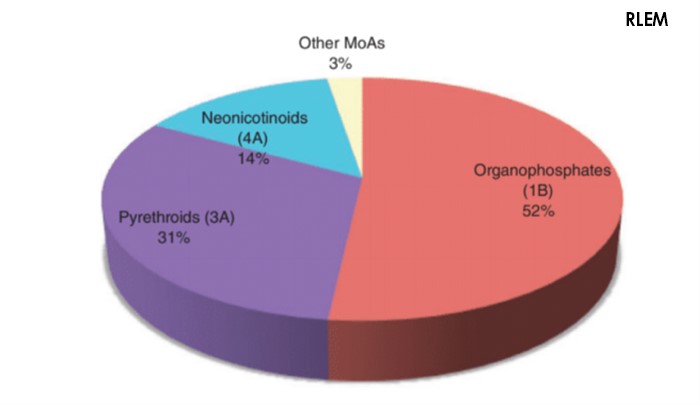
Figure 2. Percentage of pesticides used in Australia against the redlegged earth mite. (Image credit: Umina et al. 2019).
Redlegged earth mite resistance surveillance and mapping
Since the first detection of pyrethroid resistance in RLEM in 2006, resistance surveillance, supported by GRDC investment, has been undertaken on a yearly basis. This has resulted in 1029 populations being tested over the last 13 years. One hundred and ninety-five RLEM populations have now been detected with pyrethroid resistance, 59 populations have been detected with organophosphate resistance and 24 populations with resistance to both chemical groups (Table 1). Surveillance has covered a wide geographical range throughout Western and eastern Australia, covering a large portion of the entire known Australian distribution of RLEM (Figure 3).
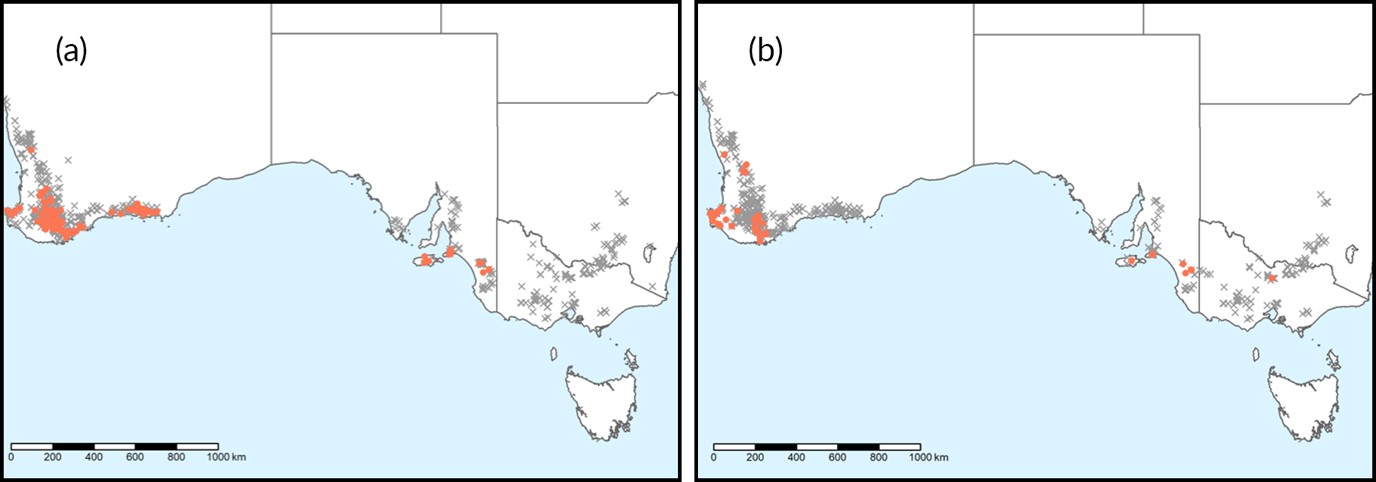
Figure 3. The distribution ofRLEM populations screened for (a) pyrethroid and (b) organophosphate resistance across Australia as of 2019. Closed circles represent populations with resistance, and grey crosses indicate populations that are susceptible to pesticides. (Image credit: Arthur et al. 2020 (Unpublished)).
Table 1. Number of H. destructor populations collected between 2006 and 2019 from Western and eastern Australia screened for resistance to organophosphates and synthetic pyrethroids.
Populations from Western Australia | Populations from eastern Australia | |||||||
|---|---|---|---|---|---|---|---|---|
Year | Sampled | With SP resistance | With OP resistance | Dual resistance | Sampled | With SP resistance | With OP resistance | Dual resistance |
2006 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
2007 | 33 | 12 | 0 | 0 | 5 | 0 | 0 | 0 |
2008 | 7 | 0 | 0 | 0 | - | - | - | - |
2009 | 46 | 12 | 0 | 0 | 3 | 0 | 0 | 0 |
2010 | 44 | 12 | 0 | 0 | 34 | 0 | 0 | 0 |
2011 | 108 | 14 | 0 | 0 | 19 | 0 | 0 | 0 |
2012 | 7 | 7 | 0 | 0 | - | - | - | - |
2013 | 8 | 6 | 0 | 0 | - | - | - | - |
2014 | 127 | 28 | 12 | 1 | 39 | 0 | 0 | 0 |
2015 | 95 | 10 | 6 | 0 | 24 | 0 | 0 | 0 |
2016 | 28 | 10 | 0 | 0 | 7 | 1 | 1 | 1 |
2017 | 119 | 22 | 8 | 4 | 47 | 6 | 1 | 1 |
2018 | 26 | 5 | 1 | 1 | 19 | 3 | 4 | 2 |
2019 | 117 | 37 | 17 | 10 | 65 | 9 | 9 | 4 |
A new GRDC investment (CES2010-001RXT) led by cesar Australia, in collaboration with the University of Melbourne and the WA Department of Primary Industries and Regional Development, will continue extensive resistance surveillance of RLEM across Australia utilising improved resistant monitoring tools such as the molecular pyrethroid resistance screening test (Edwards et al. 2018) and modelling that has been used to predict ‘at risk’ areas (Maino et al. 2018). This project will also investigate new chemical and biological options to control RLEM, as well as tools to increase confidence in seasonal RLEM risks and management options.
The role of recessive genes in managing RLEM
Resistance to pyrethroids in Australian populations of RLEM has recently been shown to be a recessive trait. In the case of recessive genetic traits, the resistant allele (allele = a version of a gene) must be carried by both parents and both copies passed on for mite offspring to exhibit resistance. If only one copy is passed on (e.g., through the maternal but not the paternal line), the offspring will remain susceptible to pyrethroids. This creates an opportunity to revert largely resistant populations to a susceptible state through management practices that allow susceptible mites (and their genes) to remain within the population. Other research by cesar Australia has shown that populations of RLEM resistant to synthetic pyrethroids have reduced fitness in comparison to susceptible populations. This is a useful insight, as the fitness cost imposed on resistant mites may help to maintain susceptible populations of RLEM (if managed correctly) as susceptible mites have a higher chance of surviving and passing on their genetic material in the absence of pyrethroid usage. Therefore, thinking about management on a genetic level is required. A strategy that involves maintaining refuges for susceptible mites is one option for maintaining a pool of susceptible alleles in a population.
Cesar Australia has investigated the possibility of strip spraying to maintain refuges of susceptible RLEM populations. Through analysis of pest migration rates and survival from pesticide applications, a simulation approach was used to design a refuge and treatment strategy to maintain field susceptibility to pyrethroids in populations with resistance. It was found that certain field configurations (e.g., treatment strip width of 50m and refuge spacing of 10m) maintained very low levels of resistance across a 30-year time horizon. This could be a successful strategy to manage RLEM as part of an integrated pest management (IPM) program. However, this novel approach will require further field validation in a variety of cropping contexts. Growers interested in trialling such novel strategies are encouraged to contact cesar Australia.
Resistance in green peach aphid
Green peach aphid is a worldwide agricultural pest that attacks a wide range of grain and horticultural crops from over 40 plant families. It is an important vector of over 100 plant viruses. This polyphagous aphid is of particular concern for canola growers in Australia due to its high transmission rate for Turnip yellows virus (TuYV) (formerly known as Beet western yellows virus). Infection of canola plants by this virus prior to stem elongation can cause yield losses of up to 50% (DPIRD, 2020). Feeding damage from high aphid densities can lead to reduced or stunted growth, while the secretion of honeydew can contribute to secondary fungal infections (Blackman and Eastop 2000; Anstead et al. 2007).
In Australian grain growing regions, control of GPA largely relies on pesticide applications and seed treatments. There are five pesticide groups currently registered for use against GPA in Australian grains: carbamates (Group 1A), organophosphates (Group 1B), synthetic pyrethroids (Group 3A), neonicotinoids (Group 4A) and sulfoximine (Group 4C) (Figure 4). Paraffinic spray oils are also registered for suppression of GPA. These chemicals are often applied prophylactically as a safeguard against infestations of this pest. This has created strong selection pressures against this pest and has contributed to the evolution of resistance in multiple Mode of Action pesticide groups (including carbamates, organophosphates, synthetic pyrethroids and neonicotinoids) in Australia.
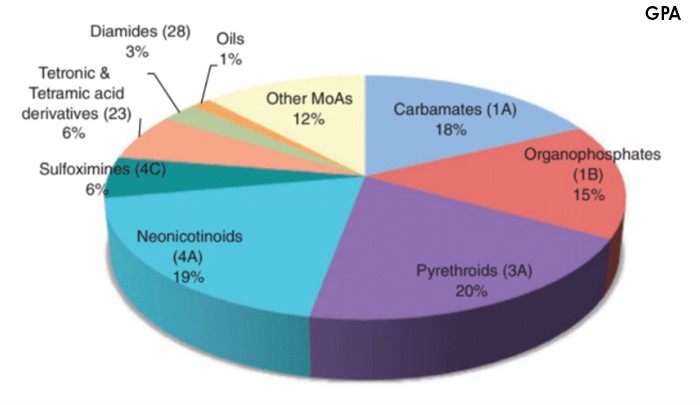
Figure 4. The percentage of pesticides used in Australia against the green peach aphid. (Image credit: Umina et al. 2019).
Resistance testing of GPA populations
Asexual reproduction in Australian GPA populations limits genetic variation. However, there are genetic differences between GPA populations. Populations that maintain enough genetic distinctiveness are termed a ‘clone’.
Recent work undertaken by cesar Australia and CSIRO as part of a GRDC investment (CES00003) has involved development of a clone database that includes information on resistance-conferring mutations. This enables researchers to track the evolution and spread of resistance across populations by identifying the genetic lineage of an aphid sample, reducing the time and costs related to resistance testing.
Within Australia, green peach aphid populations are dominated by three clones across all seasons in broadacre and horticulture cropping regions in Queensland (Qld), New South Wales (NSW), Vic, SA, WA and Tasmania (Tas). Importantly, all three clones exhibit some level of resistance to synthetic pyrethroids, carbamates, organophosphates and neonicotinoids.
Between 2015-2019, 473 GPA populations were genetically screened against known pesticide resistance conferring alleles for carbamates, organophosphates, synthetic pyrethroids and neonicotinoids. For neonicotinoid resistance testing, a subset of populations was also screened using phenotypic laboratory bioassays (Figure 5 shows maps of the populations screened for resistance to different chemical groups). This testing work identified target site resistance in almost all screened populations to carbamates and synthetic pyrethroids rendering these chemicals ineffective as a control option for GPA. The testing also detected resistance to organophosphates and neonicotinoids in GPA populations.
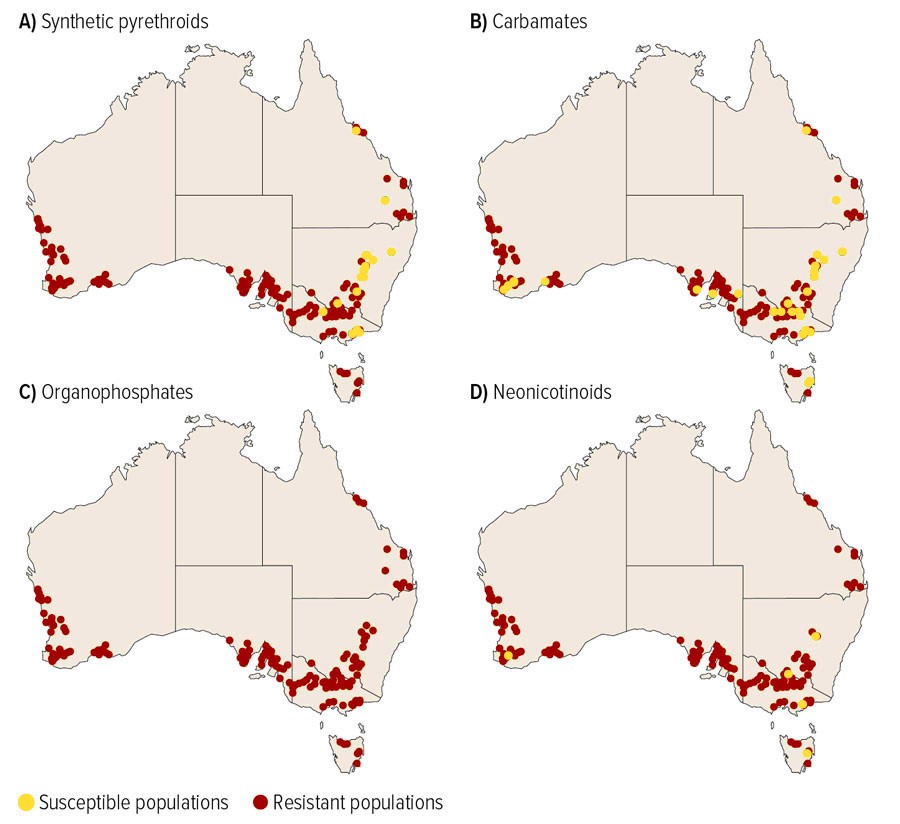
Figure 5. Resistance status of populations tested for resistance to synthetic pyrethroids, carbamates, organophosphates and neonicotinoids. The darker coloured dots represent resistant populations while the lighter coloured represent susceptible populations. (Image credit: Noakes 2020).
Resistance to organophosphates was found to be moderate in many populations and a result of metabolic resistance. With metabolic resistance, insects use enzymes to break down and detoxify pesticides, reducing their overall efficacy. As a consequence, organophosphate will provide control in some situations, but less or no control in others. Furthermore, continued use of organophosphates on such populations would likely increase their overall resistance to chemicals from this group.
Resistance to neonicotinoids in GPA is determined by an overexpression of the P450 monooxygenase CYP6CY3 detoxifying gene. This gene was only found to be expressed at low levels in the GPA populations screened, and therefore, complete chemical field failures are unlikely to occur in GPA populations with the levels of neonicotinoid resistance currently observed.
Screening for the exotic mutation; R81T in GPA populations
As part of the project, researchers also tested 181 GPA samples taken from a network of sticky traps around Australia for the gene mutation ‘R81T’. The R81T mutation has been found in GPA populations overseas and confers near total resistance to neonicotinoids when present. While the mutation was not detected in any of the tested samples, there is ongoing risk of this mutation being found in Australia either through in situ resistance evolution or importation from abroad. A risk analysis has been undertaken to identify possible incursion entry points and agricultural regions of high selection pressure.
Sensitivity shift to sulfoximines discovered in GPA populations
A sensitivity shift to sulfoximines in four different GPA populations collected around the Esperance region of WA was identified following a reported control failure in 2019. Figure 6 shows the results of bioassay testing on these populations compared with a susceptible control population. While the recorded sensitivity shifts are currently small, the possibility of GPA populations evolving further resistance to sulfoximines in the future is concerning given the small number of viable chemical options currently registered for use against GPA.
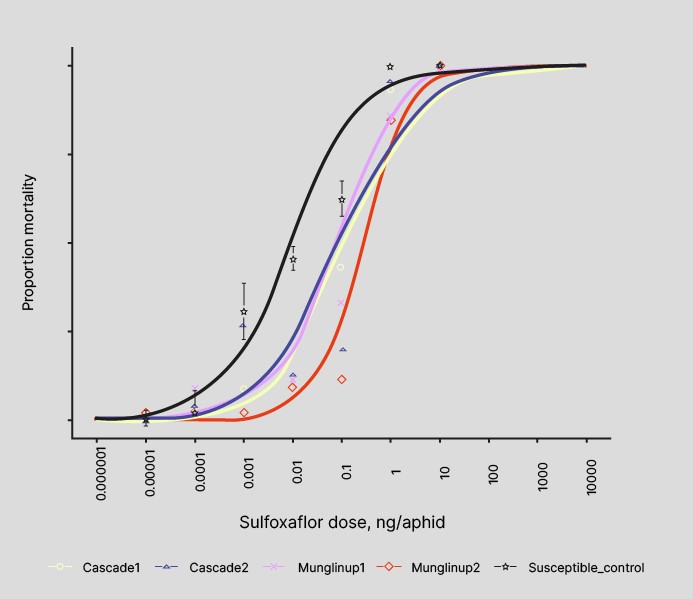
Figure 6. Dose response of sulfoxaflor for four GPA populations and the susceptible control. The populations are named according to their collection location.
Further resistance testing and novel management strategies for GPA is continuing within a GRDC investment, ‘CES2001-001RTX: Pesticide resistance in the green peach aphid: national surveillance, preparedness and implications for virus management’. This project is examining resistance trends, dispersal patterns and non-crop hosts for GPAs and associated virus risks and is developing baseline sensitivity data for new chemicals.
Bryobia mites - response to pesticides
Bryobia mites (Bryobia spp.) are important pests of winter crops and pastures. There have been growing concerns around control difficulties in the field. Management of Bryobia mites is complicated by the fact that they consist of a complex of pest species, with at least seven cryptic species present in Australian broadacre agriculture. Recent research led by cesar Australia, as part of a GRDC investment (UM00057), established baseline sensitivity data for Bryobia mites against omethoate (organophosphate) and bifenthrin (synthetic pyrethroid), two common pesticides used in the field to control this mite. Baseline sensitivity data was generated for the first time across multiple Bryobia mite populations from geographically distinct regions in Australia. The responses to bifenthrin were relatively similar across the Bryobia populations screened, however, considerable differences were evident between populations in response to omethoate. This variation appears to be linked to different Bryobia species, indicating that pesticides used against one species may not be effective against another. Unfortunately, it is impossible to differentiate species in the field. The distribution of species across Australia is currently being mapped in order to better understand the risks posed by Bryobia mites.
Conclusion
The continued spread of resistance in RLEM populations across Australia’s grain growing regions, as outlined within this paper, has led to the investigation into new ways to manage this pest, such as the role of recessive genes in maintaining susceptible RLEM populations. Furthermore, the continued spread of resistance has highlighted the importance of the resistance surveillance work for this pest, as well as the need for new chemical and biological control options, both of which will be investigated in a newly commenced GRDC investment (CES2010-001RXT).
Resistance to carbamates and synthetic pyrethroids in GPA is widespread in Australia. The recent discovery of a sensitivity shift in sulfoximines in four GPA populations in the Esperance region of WA has highlighted the need for more integrated methods of control for this pest, methods that do not rely so heavily on the limited registered chemicals available. National resistance surveillance work for GPA will continue under a newly commenced GRDC investment (CES2001-001RTX), which will also develop baseline sensitivity data to new chemicals and investigate seasonal TuYV risks.
Pesticides will continue to play an important role in RLEM and GPAcontrol, However, the increasing spread and evolution of resistance raises concerns for the long-term viability of chemical control. Future control of these pests should be in the form of an IPM approach that aims to reduce chemical usage to limit selection pressures and decrease the risk of further resistance development. Resistant management strategies (RMS) for RLEM and GPA are important resources that help maintain the effectiveness of existing chemistries.
Pesticide RMS have been developed by the National Insecticide Resistance Management (NIRM) working group for the four major resistant invertebrate pests in grains. The RLEM and GPA RMSs provide recommendations regarding effective pest management practices. In addition, a recent GRDC investment (BWD1805-006) has helped develop best management practice guides (BPMG) for RLEM and GPA, published in 2020 (Useful Resources). Growers and advisers are encouraged to become familiar with these guides and the RMSs – all freely available to download from the GRDC website.
General RMS include the following principles:
- Monitoring crops for pest and beneficial insect presence.
- Accurate pest identification to determine the appropriate control strategy.
- Utilising non-chemical control options that suppress pest populations.
- Using economic spray thresholds to guide chemical applications.
- If applying multiple pesticides within a season, rotating the chemical mode of action.
- Using selective chemicals, where possible, in place of broad-spectrum options.
- Considering the secondary impacts of chemicals to non-target pests and beneficials.
- Complying with all directions for use on product labels including using full recommended rates and good coverage of the target area to ensure the best possible chance of contact and subsequent control of the pest.
Acknowledgements
The research undertaken as part of these projects is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support.
For the green peach aphid research we also thank Corteva Agriscience, BASF, ISK, CropLife Australia, and Bayer Cropsciences for their support and acknowledge our project collaborators, CSIRO and the WA Department of Primary Industries and Regional Development (DPIRD).
For the redlegged earth mite research we would like to acknowledge our project collaborators, The University of Melbourne, WA Department of Primary Industries and Regional Development (DPIRD) and CSIRO
Useful resources
Resistance management strategy for the green peach paid in Australia grains
Resistance management strategy for the redlegged earth mite in Australia grains and pastures
Green peach aphid best management practice guide – southern
Redlegged earth mite best management practice guide – southern
Green peach aphid identification. PestBites by cesar (GPA Identification Video)
Mite identification. PestBites by cesar (Mite Identification Video)
References
Anstead, J. A., Mallet, J. and Denholm, I. (2007). Temporal and spatial incidence of alleles conferring knockdown resistance to pyrethroids in the peach-potato aphid, Myzus persicae (Hemiptera: Aphididae), and their association with other insecticide resistance mechanisms. Bull. Entomol. Res. 97: 243-252.
Blackman, R. L., and V. F. Eastop. (2000). Aphids on the world’s crops, an identification and information guide. Wiley Ltd., Chichester, United Kingdom.
DPIRD. (2020). Managing turnip yellows virus in canola. Department of Primary Industries and Regional Development
Maino, J.L., Binns, M. and Umina, P. (2018). No longer a west-side story - Pesticide resistance discovered in the eastern range of a major Australian crop pest, Halotydeus destructor (Acari: Penthaleidae). Crop and Pasture Science, 69, p 216–221.
McDonald, G., Umina, P., Lye, J., Maino, J, Perry, K. and Baker, G. (2019). Insecticide resistance in the southern region: current status, future risk and best management practices. Grains Research and Development Corporation. Canberra, Australia.
Noakes, F. (2020, 23 October). Pest detectives tracking the green peach aphid. GroundCover [Supplement – Invertebrate pest management: new frontiers], GRDC.
Ridsdill-Smith, T.J., Hoffmann, A.A., Mangano, G.P., Gower, J.M., Pavri, C.C. and Umina, P.A. (2008). Strategies for control of the redlegged earth mite in Australia. Australian Journal of Experimental Agriculture, 48, p 1506–1513.Sparks, T.C., Hahn, D.R. and Garizi, N.V. (2017). Natural products, their derivatives, mimics and synthetic equivalents: role in agrochemical discovery. Pest. Manag. Sci., 73: 700-715.
Umina, P.A. (2007). Pyrethroid resistance discovered in a major agricultural pest in southern Australia: the redlegged earth mite Halotydeus destructor (Acari: Penthaleidae). Pest Management Science, 63, p 1185–1190.
Umina, P.A. and Hoffmann, A.A. (2004). Plant host associations of Penthaleus species and Halotydeus destructor (Acari: Penthaleidae) and implications for integrated pest management. Experimental and Applied Acarology, 33, p 1–20.
Umina, P.A., Lord, A., Micic, S. and Edwards, O. (2017). Discovery and characterisation of field resistance to organophosphorus chemicals in a major mite pest, Halotydeus destructor. Pest Management Science, 73, p 1719–1724.
Umina, P.A., McDonald, G., Maino, J., Edwards, O. and Hoffmann, A.A. (2019). Escalating insecticide resistance in Australian grain pests: contributing factors, industry trends and management opportunities. Pest. Manag. Sci., 75: 1494-1506
Umina, P.A., Weeks, A.R., Roberts, J., Jenkins, S., Mangano, G.P., Lord, A. & Micic, S. (2012) The current status of pesticide resistance in Australian populations of the redlegged earth mite (Halotydeus destructor). Pest Management Science, 68, 889–896.
Contact details
Leo McGrane
lmcgrane@cesaraustralia.com
Paul Umina
pumina@cesaraustralia.com
GRDC Project Code: CES00003, UM00057, CES2001-001RTX, CES2001, CES2010-001RTX,
