Background
Pulse and pasture legumes can provide an abundant, inexpensive and sustainable source of nitrogen (N) for Australian cropping systems. Cereal yields are consistently greater following legumes due to these N inputs, and other benefits including disease and weed breaks and improvements to soil structure and biological function.
Last season (2015) 323,000ha was sown to field pea, faba bean and lentil in South Australia, accounting for 44 per cent of the national production for these three crops. These legumes, along with vetch, are nodulated by the same species of rhizobia and therefore often face similar nitrogen fixation (N2-fixation) challenges in the field.
Research at the South Australian Research and Development Institute (SARDI) is aiming to optimise N2-fixation in southern farming systems by: improving our understanding of how cultivars differ in their N2-fixation potential; clarifying where legumes respond to inoculation; identifying key agronomic factors affecting fixation; and testing new inoculants. The work has so far mainly focussed on field pea.
Cultivar variation
The total amount of N fixed by a legume is determined by both its ability to fix N and by total dry matter production. Since cultivars vary in their dry matter production, compatibility with rhizobia and tolerance of soil nitrate, it follows that their N2-fixation potential also varies considerably. In recent years, Pulse Breeding Australia (PBA) has released a number of new pulse cultivars. In the case of field pea, cultivars range from early to late season, semi-leafless shorter cultivars to conventional tall cultivars, as well as forage, grain and dual purpose cultivars.
Seven field trials have been completed in South Australia (SA)and Victoria (Vic) over the last three years to assess the N2-fixation capacity of 15 different field pea cultivars and near commercial genotypes (hereafter referred to as lines). The data will be used to develop a Symbiotic Index for field pea, so that growers can readily see which cultivars are likely to contribute most fixed N to the farming system. It will also provide pulse development programs with information on N2-fixation potential in different environments and identify opportunities for symbiotic improvement.
How much N does Kaspa field pea really fix?
Figure 1 shows the average N accumulation measured in Kaspa field pea. At peak dry matter, 132kg/ha of N had accumulated in the above ground biomass. Thirty percent of this N was derived from the soil and 70 per cent from the atmosphere (fixed in the root nodules). This corresponds to 16kg N fixed/t of shoot dry matter (DM). On average, 71kg/ha of the above ground N was removed in the grain, leaving 61kg/ha in the stubble. A further 62kg/ha is estimated to accumulate below ground in the roots, a proportion of which is also fixed. Not all N from legumes will be immediately available to the following crop as the N in the legume must first be decomposed by microorganisms and converted into mineral N. The benefits of a legume crop are generally seen for up to three years in subsequent crops.
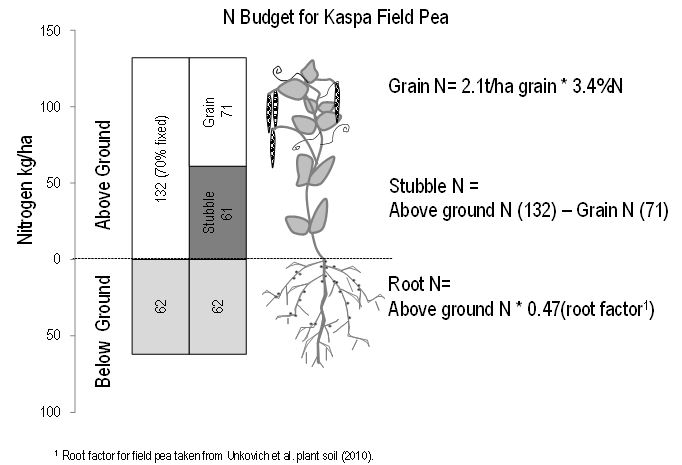
Figure 1: Average nitrogen budget for Kaspa field pea.
Comparison with other cultivars
All 15 pea lines were found to be symbiotically competent, always forming nodules and on average accumulating 128-150kg N/ha. However, the amount of fixed N remaining in the stubble varied by up to 40kg/ha (Figure 2). This is in part explained by variations in harvest index between lines, but also by differences in the percentage N (% N) they derived from fixation. Fixed N remaining in the stubble ranged from 30kg/ha for some short season and semi-leafless lines such as PBA Twilight to 60kg/ha for conventional lines such as Percy and Parafield. The forage pea Hayman generally had a high amount of N left in the stubble due to its low harvest index (70kg/ha).
In poor seasons where dry matter production is reduced and harvest index is high (e.g. dry spring) there was less difference between the pea lines, with as little as 10kg/ha fixed N remaining in stubble.
Ultimately variety selection will be based on a range of factors including rainfall, disease pressures and purpose. Nitrogen fixation may add another component to this mix or could be used to better guide future fertiliser inputs.
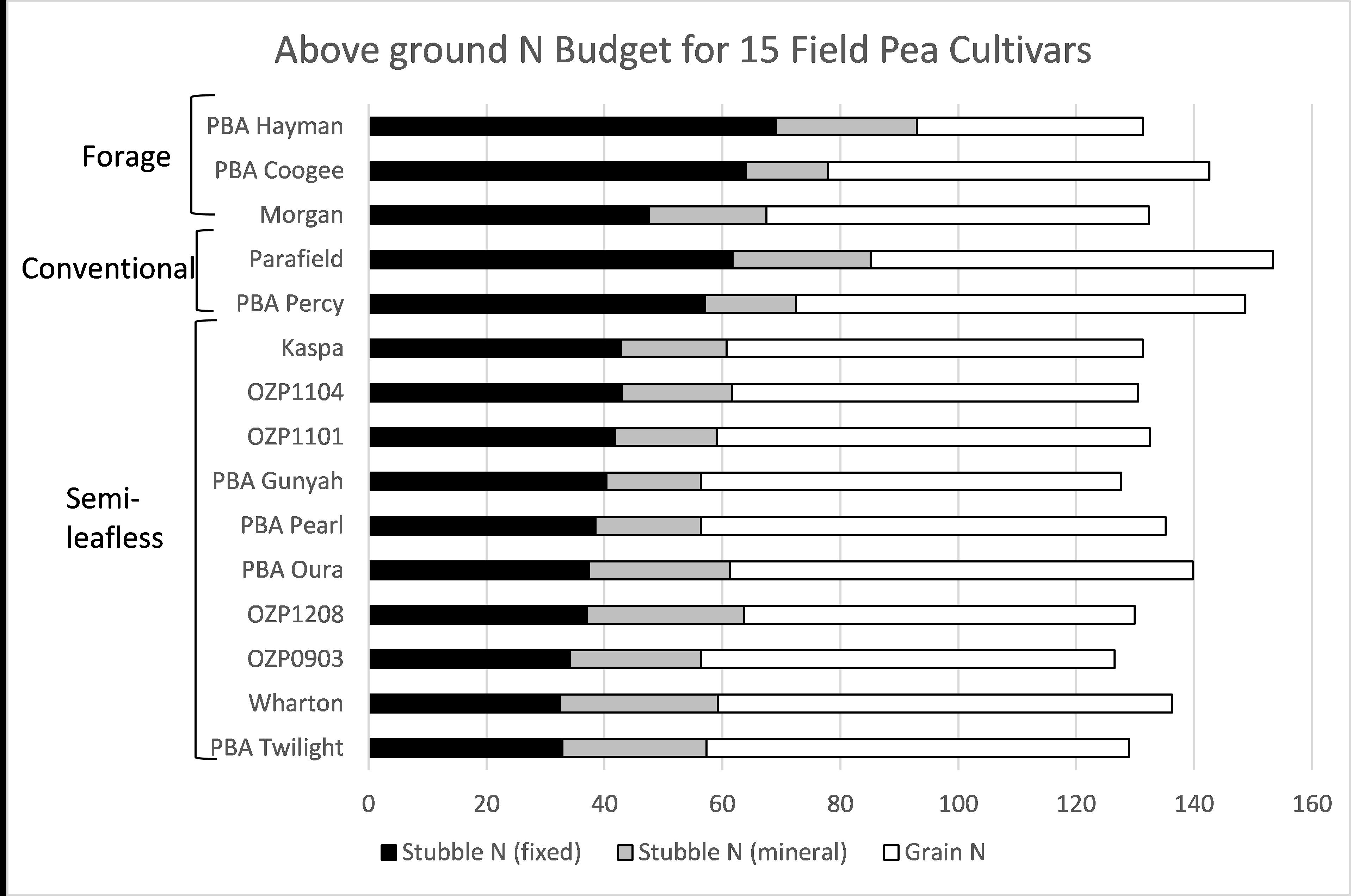
Figure 2: Above ground N budget for 15 cultivars of field pea. Data is the average of five trials (2012-2015). For Stubble N (fixed); P<0.05, lsd=11, For Stubble N (mineral); No significant differences, For Grain N; P<0.001, lsd=7.
Responsiveness of field pea to inoculation with rhizobia
Field pea, faba bean, lentil and vetch should be inoculated with rhizobia where there has been no history of these legumes and/or soil pHCa is less than 6.0. This is because suitable rhizobia are unlikely to be present in the soil. In these situations, there is a high likelihood that inoculation will increase biomass production, N2-fixation and grain yield.
Where field pea (or another legume nodulated by the same rhizobia) has previously been grown in a paddock and soil pH is neutral or alkaline, responses to inoculation with rhizobia are less likely. To what extent inoculation might still be beneficial in these situations, especially when factors such as N2-fixation are considered, has been examined in seventeen field trials using Kaspa field pea. High rates of inoculation were used to provide the best possible chance of response.
The work demonstrated that while responses to inoculation can be measured (as increased nodule mass and grain N) at sites where background rhizobia are present, they did not translate to meaningful improvement in the grain yield of field pea. Inoculation also failed to increase nodule number at many sites where nodulation arguably fell below potential, suggesting that other strategies might be needed to achieve improvements in nodulation and N2-fixation.
Site characteristics
Sites had an average pHCa of 7.1 and most were known to have previously grown field pea or another legume in the same inoculation group. As expected, all but one of the 17 sites supported substantial pre-sowing populations of pea nodulating rhizobia (median of 920/g soil, refer Figure 3). The site where no rhizobia were detected was acidic (pHCa 5.4) and had no history of field pea or similar legume having been grown.
Nodulation
The relationship between number of nodules per plant (measured approximately eight weeks after sowing) and number of pea rhizobia in the soil before sowing is shown in Figure 3. Without inoculation, the maximum number of nodules (133 per plant) was measured at a site containing approximately 10,000 rhizobia per gram of soil. Nodulation appeared to be below potential at about half the sites, but was not generally improved by inoculation. However, there was an 11 per cent increase in nodule mass (data not shown).
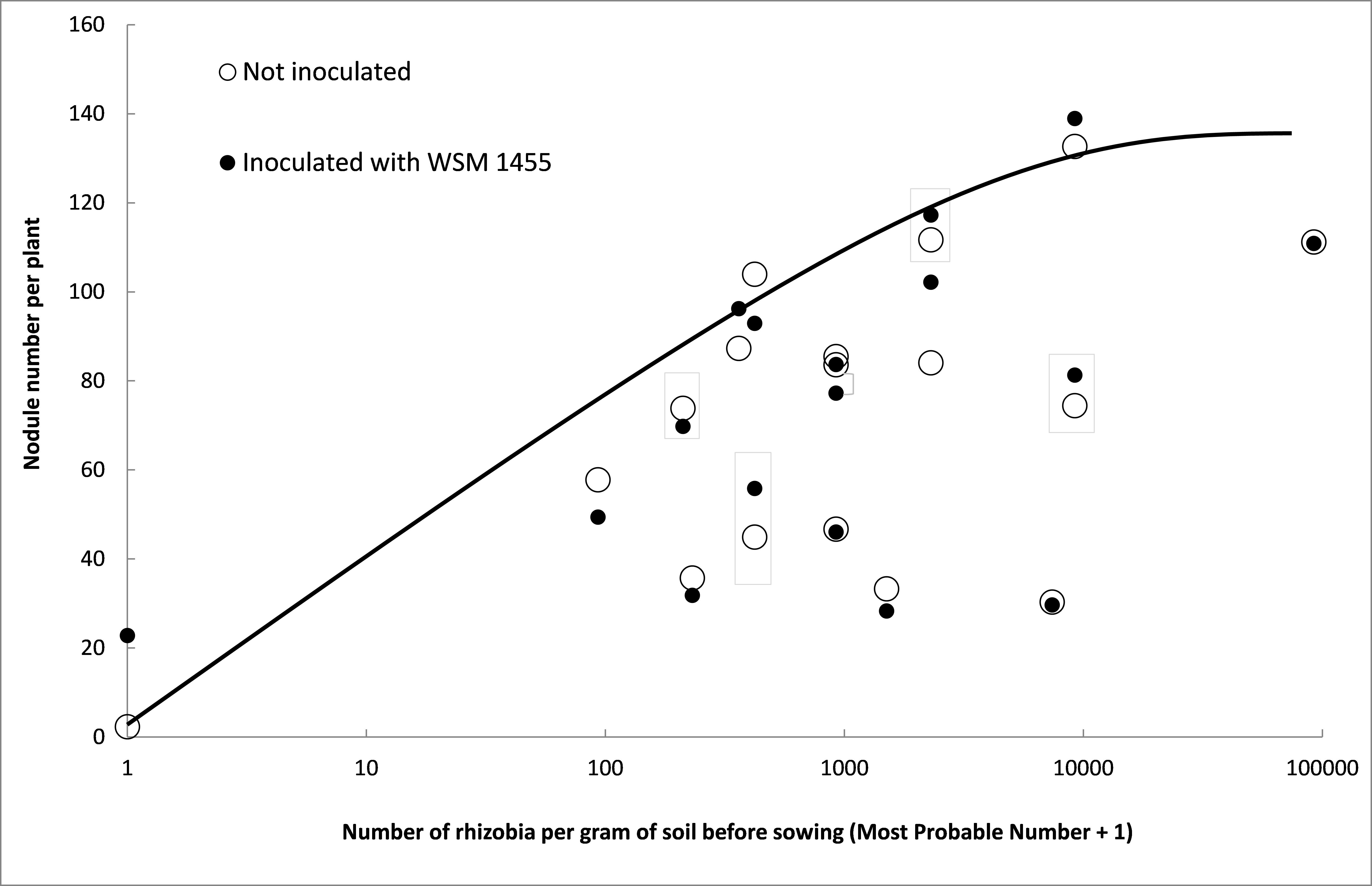
Figure 3: Effect of inoculation (closed circles) with Rhizobium leguminosarum strain WSM 1455 (Group F inoculant) on number of nodules per plant on field pea (cultivar Kaspa) at 17 field sites that varied in number of rhizobia in the soil before sowing. Unbroken line proposes the upper boundary for nodulation at the different levels of soil rhizobia.
Plant growth, N2-fixation and grain
There was limited effect of inoculation on peak dry matter production (at mid pod fill) and no effect on either N2-fixation or grain yield. However, inoculation did significantly increase (P=0.002) grain N concentration (3.5 per cent versus 3.3 per cent) and total grain N by an average of eight per cent.
There was a significant positive association between nodule number per plant and grain yield (R2 = 0.26, P <0.01) indicating there may be opportunity to improve grain yield if nodulation can be improved at some sites.
Summary of inoculation response findings
For the soils in this study that were neutral to alkaline in pH and had previously grown field pea, inoculation with rhizobia had no significant effect on grain yield, despite evidence of positive effects on nodule mass and grain N content. This work supports the inoculation guidelines for field pea described in ‘Inoculating Legumes: A Practical Guide’ that there is a ‘low likelihood of inoculation response for field pea sown on loam or clay soils with neutral or alkaline pH and a recent history of host crop’. On these soils, there would appear to be no sensible reason to inoculate field pea as an insurance policy against nodulation failure, unless there is a poor understanding of paddock history and soil pH. At this stage, we are reluctant to discount the possibility of inoculation response in faba bean since that crop produces more biomass, and therefore, has greater N demand. For lentil, which produces less biomass and has lower N demand, we anticipate the inoculation response will be similar to pea.
Inoculation response aside, the large variation in field pea nodulation and positive associations between nodule number and grain yield suggests there remains scope to improve nodulation and yield. Careful consideration of soil nutrition (in particular, N) and herbicide residues may be significant to improving nodulation.
Optimising agronomy
Agronomic practices such as time of sowing, crop nutrition and managing disease and weed pressures which optimise dry matter production of the pulse crop also drive nitrogen demand and generally encourage N2-fixation. Other factors can have more direct impacts on the symbiosis.
Mineral N at sowing
Pre-sowing mineral N in the soil (and also N applied in fertiliser at sowing) can impact on N2-fixation. High levels of mineral N in the top 10cm of soil at sowing (>30kg/ha) may reduce the number of nodules which form per plant and will reduce N2-fixation. When 30kg of N was added at sowing, nodulation was reduced by 10 per cent and N2-fixation by an average of 10kg/ha.
Herbicides
Plant back times for herbicides should be strictly adhered to. Residues from sulfonylurea (SU) herbicides are known to retard root growth and development and the ability of roots to form nodules and then fix nitrogen. In-crop herbicides which cause significant yellowing and temporary stunting of crops also have the potential to reduce nitrogen fixation of the crop (Drew et. al. 2007). These effects are thought to be more pronounced on light textured soils and when multiple stress factors are present (e.g. water, frost, SU residues).
New inoculants for the pulse industry
Strains of pea and bean rhizobia have been selected for improved nodulation at low pHCa (<5.5) at SARDI and Western Australia Department of Agriculture and Food (DAFWA). Several strains will be progressed to field evaluation in 2016. The efficacy of two non-rhizobial inoculants (actinobacteria and brevibacteria) has also been tested on field pea and faba bean with some encouraging early results.
Summary and implications
A symbiotic index for field pea lines is being developed. So far, data indicates that the amount of fixed N left behind in the stubble by the different lines after grain harvest, can vary by up to 40kg/ha.No instance of nodulation failure has been observed for any of the cultivars or advanced breeding lines of field pea.
Inoculation of field pea with rhizobia is still strongly recommended if there has been no previous history of a rhizobia host crop (pea, bean, lentil, vetch) or if soil pH is less than 6.0(Ca). In these situations, there is a strong likelihood of response to inoculation for nodulation, biomass production, N2-fixation and grain yield. At sites where good populations of pea rhizobia reside in the soil inoculation responses are more subtle and did not improve grain yield. There does however appear to be some scope to improve nodulation and presumably N2-fixation and yield at some sites. This however, will probably be achieved by managing factors such as soil mineral N and herbicide residues, rather than by inoculation with rhizobia.
Some new pea/bean rhizobia with improved acidity tolerance have been identified. Further testing on pea and faba bean on acid soils is proposed.
Contributions of fixed N from legume roots are presently not well quantified and some work has begun to gain a better understanding of these contributions and how they might be managed.
Acknowledgements
Funding was provided by GRDC project DAS00128; Optimising Nitrogen Fixation – southern region. In SA, trials were managed by the New Variety Agronomy Group (SARDI, Clare) and supported by the Hart Fieldsite Group. In Victoria, trials were managed by Jason Brand (Department of Economic Development, Jobs, Transport & Resources, Horsham). Actinobacteria were supplied by Chris Franco, Flinders University and Brevibacteria by Steve Barnett, SARDI. Acid tolerant pea rhizobia were supplied by Ron Yates (DAFWA).
Useful resources
Unkovich MJ, Baldock J, Peoples MB, 2010. Prospects and problems of simple linear models for estimating symbiotic N2 fixation by crop and pasture legumes. Plant & Soil 329, 75-89.
Drew EA, Gupta VVSR and Roget DK, 2007. Herbicide use, productivity, and nitrogen fixation in field pea (Pisum sativum).Aust. J. Agric. Res. 58, 1204-1214.
Contact details
Liz Farquharson
SARDI Soil Biology and Diagnostics
GPO Box 397, Adelaide, SA, 5001
Ross Ballard
SARDI Soil Biology and Diagnostics
GPO Box 397, Adelaide, SA, 5001



