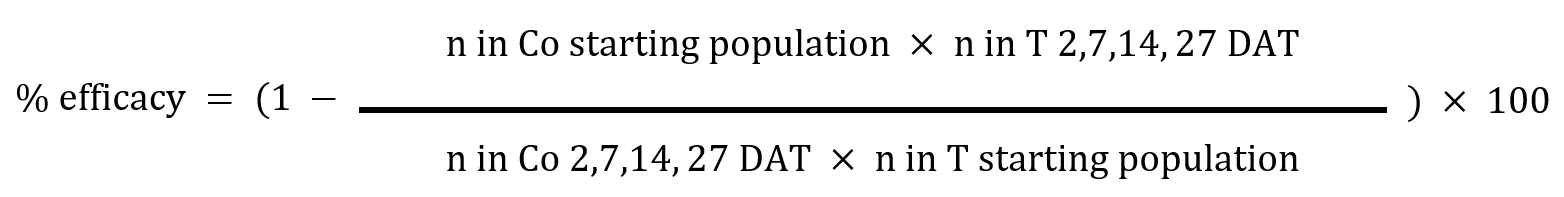Emerging aphid management issues – Russian wheat aphid distribution in northern NSW and management options for faba bean aphid
Author: Zorica Duric (NSW DPI), Mukti Chalise (NSW DPI) | Date: 29 Feb 2024
Take home message
- The Russian wheat aphid (Diuraphis noxia) has been recorded to northern New South Wales in August 2023, and its presence has been verified on wild grasses throughout the summer. Gurley (NSW) marks the northernmost location where it has been detected. Wheat, barley, barley grass, phalaris grass, and prairie grass are among the crops and grasses where Russian wheat aphid has been identified.
- The presence of the faba bean aphid (Megoura crassicauda) has been documented in Queensland, New South Wales, and Victoria. Typically, infestations occur in late winter, but in 2022, it made an unwelcome early appearance, impacting volunteer and early established faba bean crops.
- The application of tested chemicals in field conditions reduced faba bean aphid numbers compared to the control group, where intensive damage was observed along with the presence of predators and parasitoids. Both foliar treatments tested, pirimicarb and pymetrozine, were highly effective and the imidacloprid seed treatment significantly reduced aphid numbers in emerged faba beans, particularly when combined with foliar treatments.
- Typically, effective aphid management involves addressing the green bridge and volunteer plants before the season begins, monitoring both pests and beneficial insects throughout the season, and applying insecticides when aphid pressure becomes high.
Background
The Russian wheat aphid (RWA – Diuraphis noxia), a significant pest affecting cereal crops globally, was first reported in South Australia in 2016. It has since spread to the key grain growing regions of South Australia, Victoria, New South Wales, Tasmania, and Western Australia, but remains absent in Queensland. As of 2019, the northernmost confirmed presence of the Russian wheat aphid in Australia was at Tamworth (NSW). However, there were no samples positively identified as RWA in north-west NSW that would indicate the presence of the aphid in the years 2020, 2021, and 2022. The decrease in the Russian wheat aphid population during this period may be attributed to the hot and dry summer of 2019, along with the absence of an over-summer green bridge which suppressed the population prior to the wet seasons that followed. Notably, during this same period, RWA occurrences were recorded in central and southern NSW. However, in August 2023, RWA was identified in multiple locations across the northern NSW.
The initial recording of the faba bean aphid (FBA – Megoura crassicauda) in Australia dates to 2016 in Sydney, and it was later discovered in a faba bean crop in north-west NSW in 2017. Despite conducting a distribution survey, the aphid was not observed again until 2020. Subsequently, since 2020, the FBA has been consistently identified in NSW. The aphid's presence extended to Queensland in October 2021, and by 2022 outbreaks were reported in Victoria. Notably, sightings of the aphid were documented on the citizen science platform iNaturalist in late November 2020 in Victoria, preceding its confirmed spread in 2022.
Update on Russian wheat aphid activity, environmental drivers and distribution in northern NSW in 2023
While the CLIMEX model, developed by Avila et al. (2019), indicates the potential for the RWA to be established in all major Australian grain regions, its success as an invasive species depends heavily on environmental factors. Spring and autumn rainfall appear to be crucial influences on RWA population growth. The aphid is abundant in relatively warm, dry climates with summer rainfall ranging from 300 to 400 mm. Mean temperatures exceeding 20°C create an unfavourable environment for RWA and reduce survival (GRDC, 2017). However, once established in an area, the RWA is highly adaptable to environmental changes. This resilience allows it to survive at low numbers for extended periods and experience sudden population outbreaks in new areas when favorable environmental conditions are met.
Winter cereal crops typically experience infestations of RWA during warm autumn conditions, when aphids move from grasses to early planted cereals. However, in autumn 2023 in northern NSW this did not occur, with various locations, including Tamworth, experiencing significantly higher than average total rainfall. This increased precipitation may have delayed RWA movement. A further delay in the main infestation likely occurred when warm and dry conditions emerged.
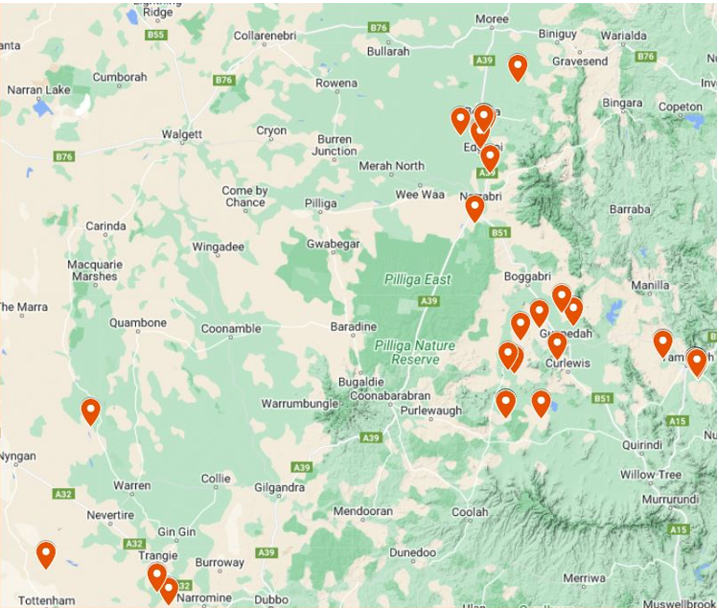
By August 2023, RWA was officially detected in several areas in the north of NSW. The first confirmation of RWA's presence for the season was documented at Tambar Springs, followed by positive samples identified in different locations. These encompassed reports received and the examination of crops and samples from commercially cultivated cereals in the Liverpool Plains, Trangie, and Moree Plains regions (Figure 1). The recorded RWA infestation has extended approximately 180 km compared to the 2019 record, reaching Gurley with no observed or reported positive findings further north.
Low numbers of RWA (1-2% of tillers infested) were detected in the crops examined in August 2023. The infestations have been detected in wheat and barley, both with and without insecticide seed treatment. Characteristic symptoms of RWA feeding were evident on the cereals, including long streaks, ranging from whitish to yellowish and longitudinal rolled leaves where the aphids were sheltered. The growth stage of the crops was approximately GS 30-32 at the time of initial infestation and inspection, except the crops in Moree Plains, which were at heading and flowering growth stages. In 2023, a total of 130 samples were inspected. Apart from the cereal hosts during the winter season, positive samples were also collected from barley grass (Hordeum leporinum), phalaris (Phalaris aquatica), prairie grass (Bromus catharticus) and regrowth of wheat and barley during summer.
Wheat, barley and durum wheat are known as primary and viable hosts for RWA for a significant part of the year. Nevertheless, the host range of RWA includes over 140 species (GRDC, 2017), suggesting the potential for RWA to sustain its population using alternative hosts such as oat, triticale, sorghum, and a diverse range of wild grasses (Duric, 2021). As the winter season concludes and temperatures rise, RWA migrates towards alternative hosts.
Wild grasses and volunteer cereals play a crucial role in maintaining RWA populations during the summer, acting as a ‘green bridge’ for infesting cereal crops in the autumn. This is because RWA relies heavily on the existence of alternative hosts, particularly barley grass, and especially in areas with relatively consistent summer watering. Other preferred alternate hosts are brome grasses, wild oat grasses, and sporadically phalaris and dactylis grasses (Pirtle et al., 2019).
Effectively managing this green bridge is crucial for controlling RWA populations. Successful control also involves monitoring cereal crops at growth stage 30 and using the developed Russian wheat aphid action threshold calculator to determine the need for intervention. By taking these proactive steps, growers can prevent RWA outbreaks and protect their crops from significant damage in later, more sensitive growth stages.
Figure 1. RWA positive samples collected in 2023 in northern NSW
Field study on faba bean aphid management
Observations from previous studies show FBA's predisposition to form hot spots within crops, primarily faba bean and common vetch (Duric et al., 2022). These hosts facilitate the aphid’s rapid reproduction, while field peas, lentils, and to a lesser extent, lucerne and subclover offer additional survival and reproductive opportunities. Notably, intensive feeding by FBA can induce damaging symptoms including necrosis, wilting, stunting, and defoliation. Furthermore, previous research demonstrates FBA's ability to vector both Bean leaf roll mosaic virus (BLRV) and Pea seed-borne mosaic virus (PSbMV), suggesting a wider potential for persistent and non-persistent virus transmission.
Previously, FBA infestations were primarily associated with late winter. However, 2022 observations in north-west NSW revealed early aphid presence in both volunteer and early established faba bean crops (Duric et al., 2023). FBA may be leveraging alternative pasture legumes as a ‘green bridge’ to connect the gap between seasons and colonise winter crops earlier. Recognising the significant challenge posed by FBA's year-round survival and migration capabilities, we conducted a field trial to assess and compare the effectiveness of various chemical control options. This initiative provides valuable insights for effective FBA control throughout the year, ensuring crop protection and minimising future losses.
Methods
This study assessed the effectiveness of various chemical products against FBA in faba beans. Faba bean seeds (Warda) were treated with imidacloprid before planting. Both treated and untreated faba bean seeds were planted on April 17, 2023 and plants germinated on May 1, 2023. Additional foliar insecticide treatments (pirimicarb or pymetrozine) were applied later, after aphids had established. Six treatment groups in total were compared: untreated control (Con), imidacloprid seed treatment only (Imi), pirimicarb foliar spray (Pir), pymetrozine foliar spray (Pym), combined imidacloprid seed treatment and pirimicarb spray (Imi/Pir), and combined imidacloprid seed treatment and pymetrozine spray (Imi/Pym). The experimental design was a randomised complete block with four replicates. The rates used are given in grams of active ingredient (g a.i.) applied per ha (Table 1).
Table 1. Descriptions of insecticides used in the experiment and application rate in field conditions
Active ingredient (a.i.) | Formulation | Application rate | Recommended field rate | Type of pesticide | Critical use comments |
|---|---|---|---|---|---|
Imidacloprid | 600 g/L | 120 mL/100 kg of faba bean seed | 4A group Insecticide | Do not graze or cut for stock feed within 16 weeks of sowing. | |
Pirimicarb | 800 g/kg | 128 g a.i./ha | 160–190 g/ha | 1A group Insecticide/ Aphicide | Minimum retreatment intervals 14 days. Do not apply more than 2 applications per crop. Withholding Period: |
Pymetrozine1 | 500 g/kg | 100 g a.i./ha | 200 g/ha for faba bean aphid | 9B group Insecticide | Minimum retreatment interval 14 days. Withholding Period: Harvest: Not required when used as directed. Grazing: Do not graze or cut for stock food for 21 days after application Do not apply after BBCH 70 Do not apply more than 2 applications per crop. |
1Permit no PER85363 allows minor use of pymetrozine in faba beans until 31 August 2026 for control of specified aphid species. The permit is valid in all states and territories except Victoria.
Prior to the field experiment, FBA colonies were multiplied in insect cages under controlled glasshouse conditions. When faba bean plants emerged and reached the ‘three pairs of unfolded leaves’ growth stage, repeated infestations were carried out to establish aphid colonies on them. Ten randomly chosen plants were used within each plot to count live adults and nymphs (Day 0). Foliar insecticides were then applied on the same day. Subsequently, the same labelled plants were monitored on days 2, 7, 14, and 27 after foliar treatment (except for imidacloprid seed treatments, where data refer to days after infestation).
To investigate the impact of insecticide treatment on both aphid survival and their offspring production, a linear mixed-effects model (LMM) was employed. To identify specific treatment groups with significant differences in aphid survival and progeny production, pairwise Least Significant Difference (LSD) tests with Bonferroni adjustment were conducted. Additionally, Henderson-Tilton’s formula was used to calculate the percentage efficacy of the treatments against FBA adults:
n = adult aphid numbers, T = treated, Co = untreated, DAT = days after foliar treatment
(for imidacloprid seed treatments, data refer to days after infestation).
Results and discussion
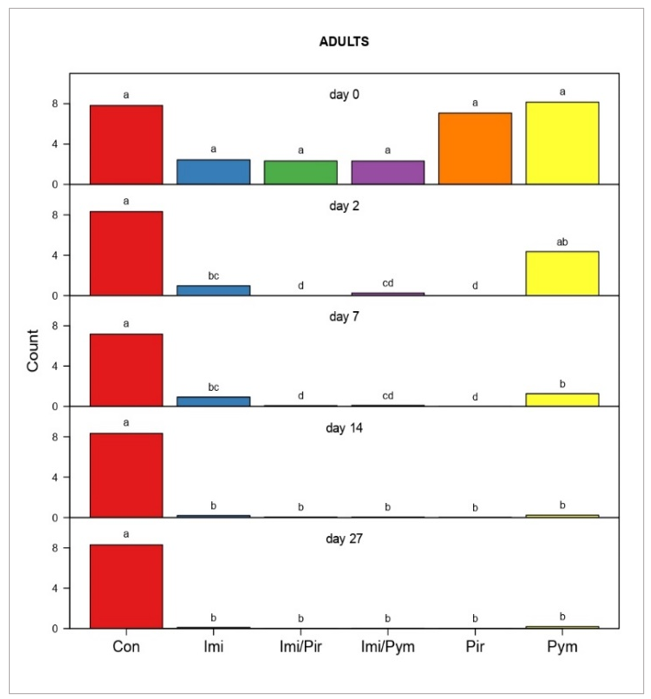
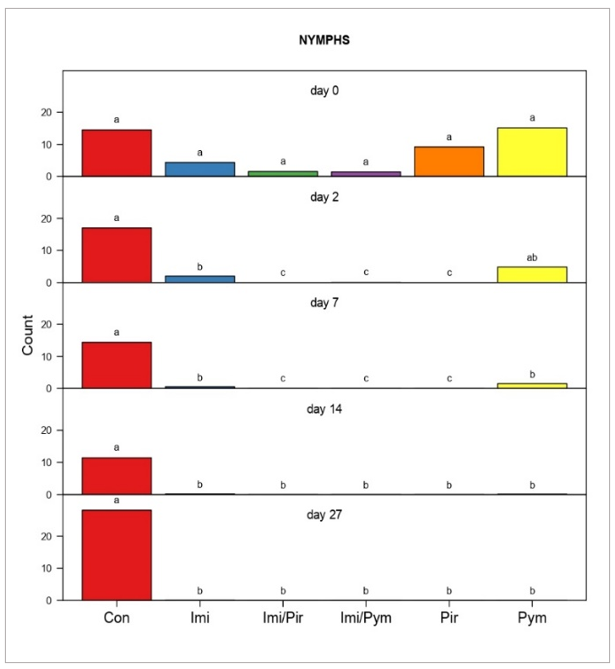
Aphid colonisation and establishment on imidacloprid seed-treated plants was less than on seed-untreated plants at day 0 (Figures 2 and 3), however there was no significant difference in their initial levels across the treatments. All insecticide treatments, including imidacloprid seed treatments, resulted in consistent reductions in aphid populations on days 2, 7, 14, and 27. Significant differences were evident in all insecticide treatments within each pairwise comparison of FBA adults (Figure 2) and nymphs (Figure 3), except on day 2, where the pymetrozine treatment was not significantly different to the untreated control for both adults and nymphs. In contrast to all treatments, populations in the control treatments either remained stable in adult counts or gradually increased in nymph counts until the final observation on day 27, with a slight decrease on day 14 likely attributed to environmental conditions.
Control plots suffered severe damage, with honeydew, necrosis, and stunted growth. Notably, a new symptom was observed: a curving of the stalk's top part where aphid colonies flourished (Figure 4). While beneficial predators like white collared ladybirds (Hippodamia variegate) were found on these large control colonies, no such presence was noted in the insecticide-treated plots. Moreover, mummified FBA were identified for the first time, a valuable discovery given the limited knowledge about FBA predation and parasitism (Figure 5).
Figure 2. Pairwise comparisons of faba bean aphid adult survival following insecticide treatments. Data represents survival observed on Day 0 and Days 2, 7, 14, and 27 after foliar treatment (for seed treatments with ‘imidacloprid’ data refers to days after infestation).
The model treated ’day’ and ’treatment’ as fixed effects, and ’repetitions’ as a random effect. Data within rows marked by the same lower case letter did not differ significantly at the 5% level of significance.
Figure 3. Pairwise comparisons of faba bean aphid nymph production following insecticide treatments. Data represents production observed on Day 0 and Days 2, 7, 14, and 27 after foliar treatment (for seed treatments with ‘imidacloprid’ data refers to days after infestation).
The model treated ’day’ and ’treatment’ as fixed effects, and ’repetitions’ as a random effect. Data within rows marked by the same lower case letter did not differ significantly at the 5% level of significance.
Figure 4. Faba bean plant infested with aphids, causing curving of the stalk.
Figure 5. Mummified aphid found in faba bean aphid colony.
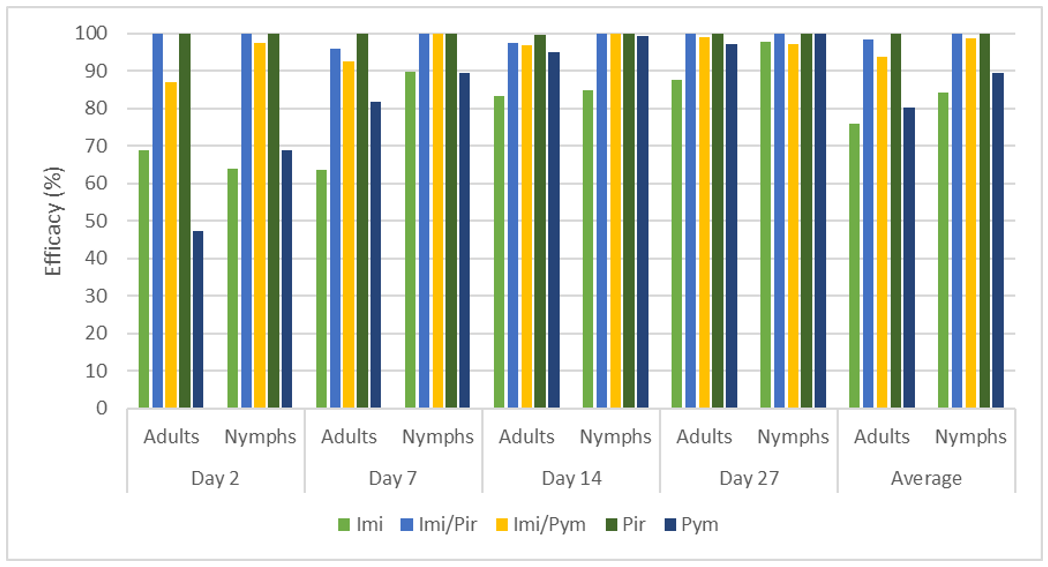
The low aphid population established on Day 0 likely explains the moderate efficacy of imidacloprid seed treatment at Day 2 (68.9% for adults, 63.8% for nymphs). However, its efficacy increased by Day 14, five weeks after the germination of faba beans, reaching 87.4% for adults and 97.9% for nymphs (Figure 6). This delayed but potent suppression, supported by our previous glasshouse experiments (Duric et al., 2023), highlights the potential of imidacloprid to prevent early infestations and effectively control FBA aphid populations at the beginning of the winter season.
Data analysis confirmed that all foliar and combined foliar-imidacloprid seed treatments effectively suppressed FBA populations, preventing re-infestation in later observations. As observed in our previous study (Duric et al., 2023), pirimicarb demonstrated exceptional efficacy, averaging 99.9% for adults and 100% for nymphs, with similar results in the pirimicarb-imidacloprid combination (Figure 6). This aligns with our past findings and highlights its effectiveness. However, it is crucial to acknowledge that new research (Knapp et al., 2023) raises concerns about pirimicarb's toxicity to beneficial insects like parasitoid wasps, key biological control agents for aphids.
Compared to our previous observations of slower action in glasshouse experiements (Duric et al., 2023), in this field trial, pymetrozine demonstrated an increase in efficacy by Day 2 (47.4% for adults, 68.9% for nymphs). This rapid onset could be attributed to environmental factors in the field setting. The insecticide maintained its potency throughout the study, reaching 97% and 99.8% control for adults and nymphs, respectively, by Day 27. Combined with seed treatment, its average efficacy remained consistently high at 93.9% for adults and 98.7% for nymphs.
Figure 6. Efficacy (%) of insecticides on faba bean aphid adults and nymphs 2, 7, 14 and 27 days after foliar treatment (for seed treatments with ‘imidacloprid’ data refers to days after infestation).
Conclusion
RWA is present in the northern region and has not been detected north of the Moree plains. Late winter surveys confirmed RWA presence on cereal crops exhibiting characteristic symptoms. Following its spring migration, RWA has also been found on volunteer cereals and wild grasses like barley grass, phalaris, and prairie grass during the late spring and summer months. To effectively manage RWA, controlling volunteer cereals and the wild grasses before autumn planting is crucial to prevent early infestations. Additionally, monitoring RWA populations in cereals during early growth stages allows time for implementing control measures if necessary.
The results of the field study on the most effective insecticide treatments for managing FBA correlate well with our previous glasshouse study. Both imidacloprid seed and foliar insecticides, pirimicarb and pymetrozine, alone and combined treatments, reduced aphid populations with significant differences except for the pymetrozine treatment on day 2. No re-infestation occurred in any treatment during the experiment, while the colony was increasing in the control treatment. The presence of predatory species and parasitised aphids was recorded in control treatments.
Generally, effective aphid management should involve managing the green bridge and volunteer plants before the season begins, monitoring both pests and beneficial insects during the growing season, and applying insecticides based on economic thresholds whenever feasible. It is crucial to implement a rotation of chemicals throughout the growing season, opting for products with diverse modes of action, and considering options with less impact on beneficials when available.
References
Avila GA, Davidson M, van Helden M and Fagan L (2019). The potential distribution of the Russian wheat aphid (Diuraphis noxia): an updated distribution model including irrigation improves model fit for predicting potential spread. Bulletin of Entomological Research 109, 90–101.GRDC (2017).
Duric Z (2021). How wide is the distribution of RWA in northern NSW and is sorghum an alternative summer host?, GRDC Update paper.
Duric Z, George J, van Leur J (2022). Megoura crassicauda Mordvilko (Hemiptera: Aphididae), a potential threat to Faba bean industry in New South Wales, Gen. Appl. Ent. 50: 11-17.
Duric Z, Gillard G, Chalise M (2023). Aphids in faba beans - an update with a review of management strategies of faba bean aphid, GRDC Update Paper.
GRDC (2017) Tips and tactics Russian wheat aphid.
Knapp R, Mata L, Umina P, Miles M, Hoffmann A, Lowe L (2023). Minimising the impact of insecticides on beneficials in broadacre crops, GRDC Update Paper.
Pirtle E, Maino J, Lye J, Umina P, Kirkland L, Heddle T, van Helden M (2019). Current management advice for Russian wheat aphid and Green peach aphid, GRDC Update paper.
Russian wheat aphid action threshold calculator
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. The authors wish to acknowledge the collaboration with growers and agronomists during Russian wheat aphid surveys. Special thanks to Karlijn Spaans and Stuart Marshman for technical support, and Steven Harden (all DPI) for statistical support.
Contact details
Zorica Duric
Department of Regional NSW
4 Marsden Park Rd, Tamworth, 2340, NSW
Ph: 02 6763 1154
Email: zorica.duric@dpi.nsw.gov.au
Date published
February 2024
GRDC Project Code: CES2204-001RTX,
Was this page helpful?
YOUR FEEDBACK