A foe in the fallow: what happens with Fusarium crown rot between seasons?
Author: Toni Petronaitis (UNE / NSW DPI), Clayton Forknall (DAF Qld), Steven Simpfendorfer (NSW DPI), Richard Flavel (UNE), David Backhouse (UNE) | Date: 01 Mar 2024
Take home message
- Cereal varieties with better partial resistance can still experience significant (up to ~6-fold) increases in Fusarium pseudograminearum (Fp) colonisation after senescence (crop maturity)
- Colonisation of cereal stubble by Fp after harvest can be maintained at high levels for at least 1 year under natural field conditions
- Post-harvest colonisation of cereal stubble by Fp could contribute inoculum for future seasons, particularly if infected stubble is disturbed and redistributed e.g., via harvest of a shorter-stature break crop (e.g., chickpea)
- Lowering the harvest height of cereal crops infected with Fp can prevent colonisation of retained stubble after harvest and may be a useful management strategy in high-risk scenarios.
Introduction
Fusarium crown rot (FCR) is a chronic disease of cereals in Australia and causes significant damage to infected crops through yield loss and reduced grain quality. In the northern region (northern NSW and Qld), the disease is primarily caused by the fungus Fusarium pseudograminearum (Fp), but F. culmorum and F. graminearum can also cause FCR. These fungi can survive three or more years in cereal stubble (Summerell and Burgess 1988), which has become increasingly problematic due to cereal stubble retention.
Recent research has confirmed that Fp can also continue to colonise cereal stubble after harvest, known as saprotrophic colonisation. Over a 6-month summer fallow, saprotrophic colonisation by Fp resulted in a 60 to 70% increase in the height that Fp was detected in standing stubble at two sites in northern NSW (Petronaitis et al., 2022). Post-harvest colonisation of stubble by Fp may therefore contribute to the build-up of FCR inoculum in stubble-retention systems.
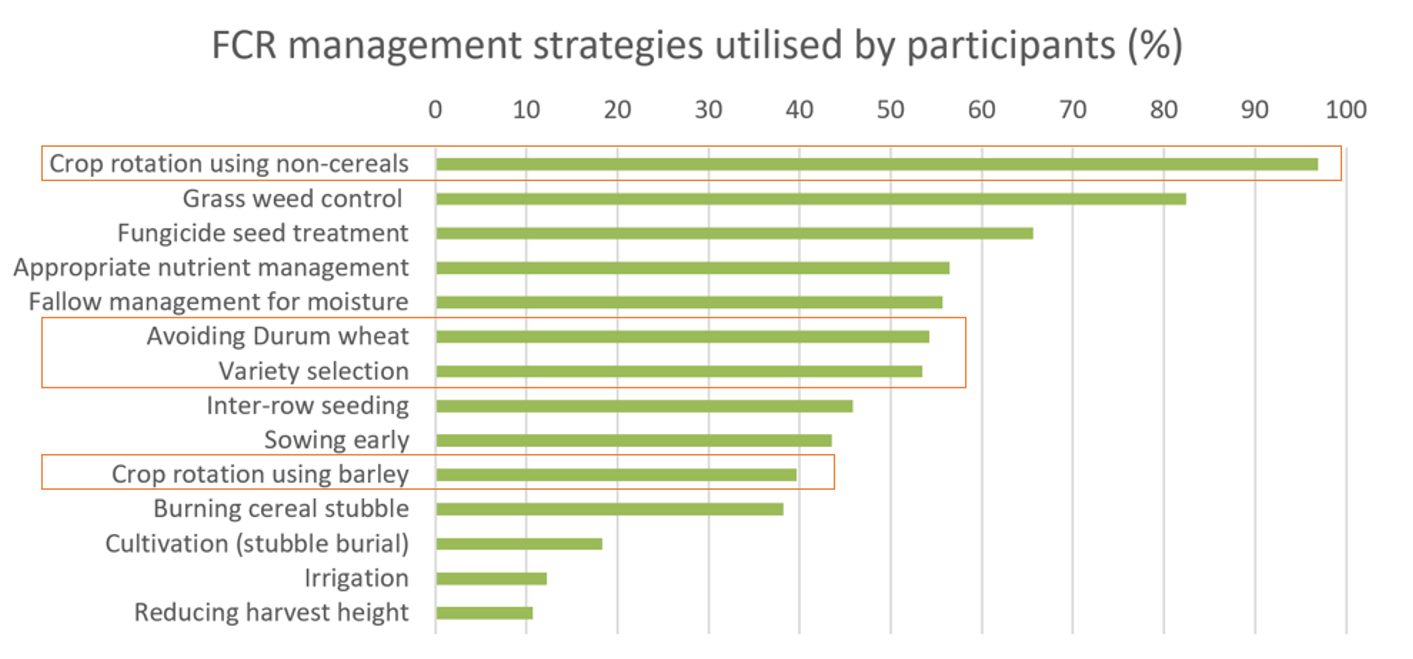
Saprotrophic colonisation of cereal stubble by Fp has not been well-characterised. During plant development, Fp will colonise stems more aggressively in hosts which are more susceptible to FCR (e.g., durum wheat) (Knight and Sutherland 2017). This can lead to more inoculum accumulation during the growing season. It is unknown whether aggressive colonisation continues after harvest in more susceptible hosts, and whether using cereals with improved levels of genetic resistance provides any subsequent inoculum benefit (e.g., by slowing or preventing Fp colonisation of stubble). This is important to investigate, as crop and variety selection are among the most popular strategies that growers and advisors use to manage FCR (Figure 1).
Figure 1. Strategies used in the last 2 years to manage FCR by 130 participants who completed the Fusarium crown rot survey: a grower and agronomist questionnaire conducted in August 2023 under the GRDC and DPI co-investment (DPI2207-004RTX). Strategies that involve crop and variety selection are circled. Participants include growers and agronomists from Qld, NSW, SA, and Vic.
Glasshouse experiment method
Ten cereal cultivars with a range of FCR ratings (Table 1) were selected and the movement of Fp tracked within the stems, from seedlings through to post-harvest stubble.
Table 1. Ten cultivars were selected for the study based on their relevance to the northern region and covering a range of crop types and FCR resistance ratings.
Cultivar name | Crop species | FCR resistance rating |
|---|---|---|
LRC2012-122 | Bread wheat | MR–MS to MR1 |
LRPB Hellfire | Bread wheat (APH) | MS–S |
LRPB Lancer | Bread wheat (APH) | MS–S |
LRPB Stealth | Bread wheat (APH) | S |
LRPB Kittyhawk | Dual purpose wheat (APH)2 | S–VS |
DBA Lillaroi | Durum wheat | S–VS |
Oxford | Barley | MS–S |
Commander | Barley | S |
Spartacus | Barley | S |
Eurabbie | Oat | NA |
1 Germplasm and FCR rating kindly supplied by Cassy Percy, University of Southern Queensland, 2021. Abbreviations: Australian Prime Hard (APH), Durum Breeding Australia (DBA), Fusarium crown rot (FCR), Leslie Research Centre (LRC), Longreach Plant Breeders (LRPB), moderately resistant (MR), moderately susceptible (MS), not applicable (NA), susceptible (S), very susceptible (VS). | ||
The experiment was conducted in a glasshouse at Tamworth Agricultural Institute (Tamworth, NSW). Two seeds of an individual cultivar were placed in grow bags containing potting mix and covered with a 2 cm layer of Fp grain inoculum-potting mix combination (1% grain inoculum by weight). Plants were thinned to one plant per bag after 10 days. Plants were assessed at four sampling times (in days after planting, or DAP) at various targeted growth stages (GS): stem elongation (80 DAP, GS32), anthesis (113 DAP, GS61), maturity (147 DAP, GS90), and post-harvest (166 DAP, GS90 + 2 weeks), the latter following regular moisture treatment. Plants were washed and rated visually for severity of FCR (stem browning). The main tiller was retained for culturing, and any remaining tillers were dried at 30°C for 24 hours and submitted to the South Australian Research and Development Institute (Adelaide, South Australia) for qPCR analysis of Fp DNA.
Main tillers were surface sterilised using 5% sodium hypochlorite solution (5 mL sodium hypochlorite solution, 45 mL distilled water, 50 mL > 98% ethanol) for 1 minute then washed three times with sterile reverse osmosis water and dried for 2 hours in a laminar flow. The tillers were aseptically trimmed into 1 cm segments and numbered sequentially starting from the crown. Segments were cultured on 1/4 potato dextrose agar (PDA) + novobiocin and incubated under alternating white and near ultraviolet lights for a 12 h photoperiod of 66.6% alternating fluorescent (FL36W/865, Sylvania, East Sussex, United Kingdom) and 33.3% blacklight blue (F36T8 BLB, Crompton lighting, Bradford, United Kingdom) for 5 days at 25°C. The incidence of stem colonisation was determined by scoring each tiller section for the growth of Fp based on colony morphology. Maximum colonisation was defined as the highest tiller section at which Fp was detected within the tiller and reported as a height (in cm).
The factorial combination of treatments, being all combinations of cultivar and sampling time, were assigned to tubs according to a split-plot design. In this case, sampling times were randomly assigned to main plots, where a main plot was defined as a group of 10 grow bags arranged in a 2 x 5 configuration. Using this configuration of grow bags, and due to the size of the tubs, two main plots occurred within each plastic tub. Cultivars were randomly assigned to individual grow bags within main plots. All treatment combinations were replicated six times.
The response variable, maximum height of colonisation, was analysed using a linear mixed model framework, whereby cultivar, sampling time and their interaction, were fit as fixed effects, while terms describing the experimental design structure were fit as random. The model was fit using the ASReml-R package in the R statistical computing environment.
Glasshouse experiment results – what did we find?
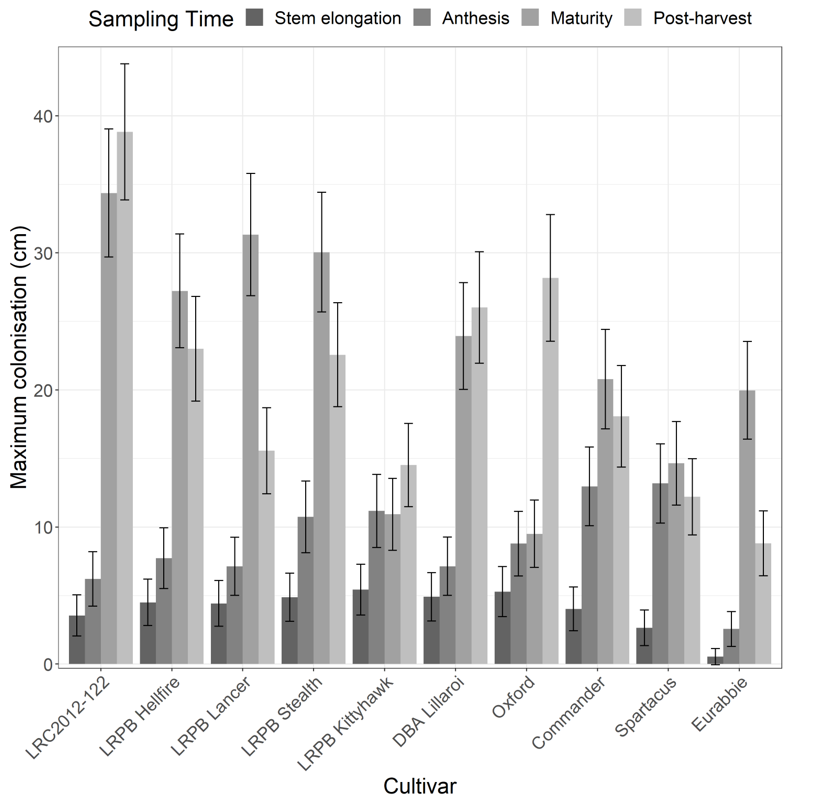
Analysis of maximum colonisation revealed a significant interaction between cultivar and sampling time. During the growing season (stem elongation and anthesis), Fp did not colonise as high up main tillers in oat var. Eurabbie compared with most of the other cultivars (Figure 2). This was the only case in which host resistance to FCR significantly suppressed Fp colonisation in the living plant. By maturity, improved genetic resistance did not appear to suppress colonisation by Fp in any of the cultivars tested (including oat). Some of the cultivars with better FCR resistance ratings experienced the highest increase in Fp colonisation, for example LRPB Lancer (~4-fold increase) and LRC2012-122 (~6-fold increase) (Figures 2 and 3). As such, Fp colonisation of stems around the time of harvest did not relate comparatively with the reported host resistance ratings of the different cultivars tested.
Figure 2. Maximum vertical colonisation (cm) of the main stem of different cereal cultivars by Fp at four different time points: stem elongation, anthesis, maturity, and post-harvest. Error bars represent the approximate back-transformed standard error of the mean.
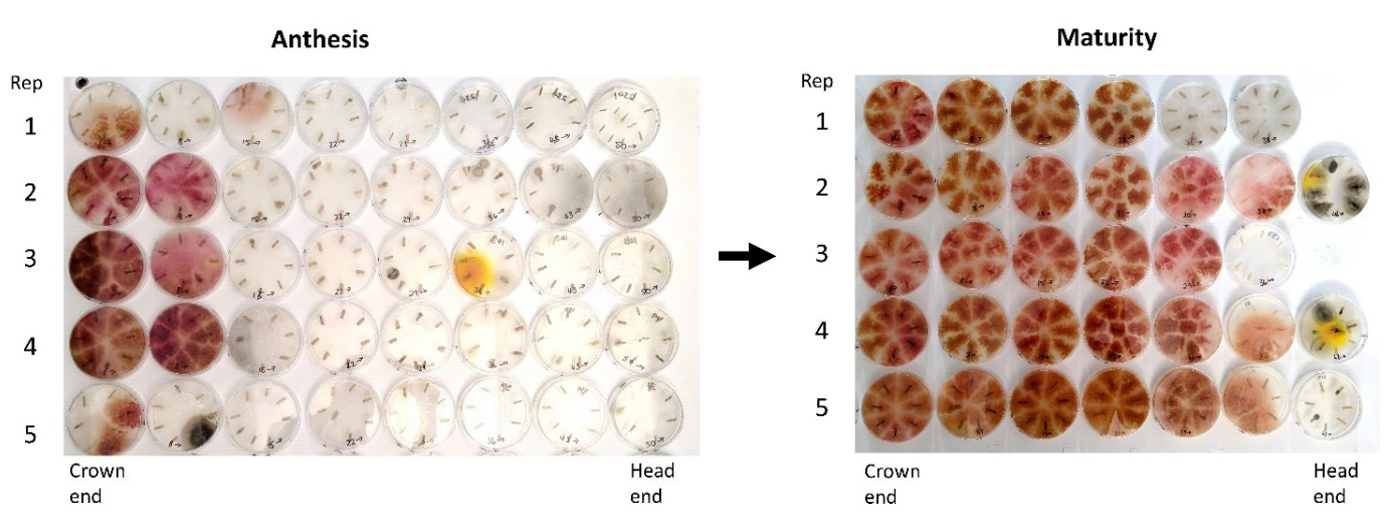
Figure 3. Example of the large increase in Fp colonisation observed between anthesis and maturity in the partially FCR-resistant wheat germplasm LRC2012-122. The Fp colonies appear as dark patches surrounding the stubble pieces (colonies are red when printed in colour). This shows extensive colonisation of the plant by Fp following plant senescence. Samples are representative of all six replicates in the experiment.
The Fp DNA levels and crown rot index (CRI) results aligned well with the FCR ratings (data not shown), confirming that we achieved the desired range of different infection and disease severity levels. Interestingly, the MR–MS to MR line LRC2012-122 had relatively low Fp DNA and CRI, but the highest height of Fp colonisation at maturity and post-harvest. Conversely, the two cultivars with the highest Fp DNA levels and CRI (S-rated barley cv. Commander and S–VS wheat cv. LRPB Kittyhawk) were among the cultivars with the lowest height of Fp colonisation from maturity onwards (Figure 2). These results showed that lower levels of stem colonisation (determined via culturing) did not translate to lower DNA levels or disease symptoms in the plant, and vice-versa. This could be explained by more susceptible cultivars accumulating higher levels of DNA in-season due to aggressive colonisation by Fp (Knight and Sutherland 2017), which is not experienced during saprotrophic colonisation.
Additional watering of the stubble between maturity and post-harvest did not consistently increase saprotrophic colonisation by Fp. This is contrary to prior work, which showed the FCR pathogen can colonise sterile cereal stubble at a rate of up to 1 cm/day under consistently high humidity (Petronaitis et al., 2020). In the field, Fp can increase by up to 21 cm (or to the final cut height of stubble) over a 6-month summer fallow (Petronaitis et al., 2022). We suspect further saprotrophic colonisation might have been detectable via culturing in the present study if the post-harvest period had been extended beyond 2 weeks. The good news is that there may not be significant change in Fp colonisation during shorter periods, e.g., a harvest window. This may allow for additional time to test Fp levels in stubble and/or manage, if needed, to prevent further post-harvest colonisation.
Crop/variety selection is still a useful tool for managing FCR
The glasshouse experiment supports the use of cereal cultivars with partial resistance as part of an integrated management strategy for FCR. The more (partially) resistant cultivars were generally associated with a reduction in Fp DNA and disease severity, which can protect against yield and quality losses to FCR. The preliminary results from the FCR questionnaire show that growers and agronomists already employ this strategy frequently. Further education of industry is still required about which crops and varieties are most suitable, as almost 40% of participants indicated they have implemented a barley in their rotation in the last 2 years to reduce FCR risk. However, barley is susceptible to FCR, and exhibited the largest Fp DNA accumulation of all crop types in the glasshouse experiment. The general earlier maturity of barley compared with wheat can reduce exposure to heat and/or moisture stress during grain filling. This can be protective against FCR expression and associated yield loss from FCR in barley but is not guaranteed to reduce Fp inoculum levels.
Infection of more FCR-resistant cultivars by Fp can be difficult to detect visually, as basal browning symptoms are milder. Growers may therefore be unaware of Fp infection and/or the extent of colonisation in crops with minimal FCR symptoms. In our study, oat var. Eurabbie had lower (but still detectable) basal browning symptoms compared with barley and wheat cultivars. Oat may therefore be affected by FCR more frequently than previously thought, with visible detection via basal browning possible. The oat also contained similar levels of Fp DNA compared to several of the bread wheat cultivars in our experiment. Oat is therefore not recommended as a break crop for the purpose of reducing FCR risk within a cropping sequence. Still, oat crops may present a more diverse option for managing FCR as the stubble can decompose more rapidly than wheat and barley (to potentially displace Fp). It may also have the advantage of being grazed by stock which can also reduce stem colonisation by FCR pathogens (Nelson and Burgess 1995).
What are the dangers of post-harvest colonisation of stubble?
Saprotrophic colonisation of cereal stubble by Fp could contribute additional inoculum for future seasons. This is particularly important given that less-susceptible cereal crop types/cultivars can still experience extensive colonisation by Fp after harvest. In the glasshouse study, it appeared that plants which were less affected by FCR (e.g., oat and LCR2012-122) experienced higher levels of saprotrophic colonisation – possibly because the plants were able to grow taller and healthier due to improved partial resistance to FCR. This additional Fp inoculum, which is often not accounted for in integrated disease management, may be contributing to the persistence of FCR within cropping systems.
Previous work has shown that Fp can persist for at least 12 months in upper parts of cereal stubble that have been saprotrophically colonised after harvest (Petronaitis et al., 2022). Inoculum maintained long-term in this section of the stubble may become problematic if standing stubble is disturbed, perhaps by being knocked over or distributed prior to sowing a new cereal crop. Examples of this include light tillage (e.g., Kelly chaining) or when harvesting a shorter stature break crop (e.g., chickpea or lentil) which have been sown into cereal stubble infected by Fp. This is because the infected stubble is likely to be spread at harvest when collecting pods low to the ground. Reducing the height of infected cereals at the time of cereal harvest can prevent saprotrophic colonisation (Petronaitis et al., 2022). This strategy may be useful in high FCR risk scenarios where shorter-stature break crops (e.g., chickpea and lentil) are planned in the rotation, to prevent the spread of Fp inoculum during break crop harvest.
Further field evidence of saprotrophic colonisation by Fp
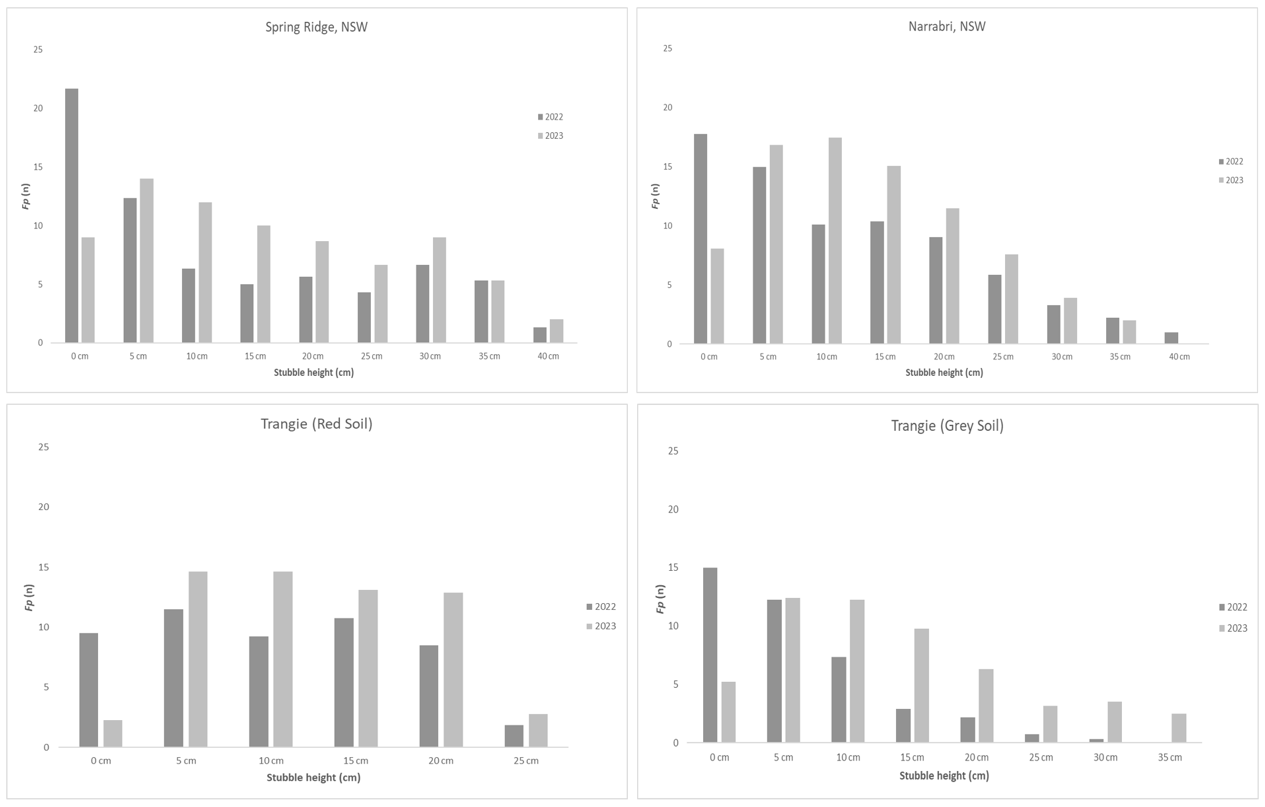
Preliminary data from Northern Farming Systems (DAQ2007-002RTX) trials at four sites in northern NSW have provided further evidence of saprotrophic colonisation of post-harvest stubble by Fp. Stubble naturally infected with Fp was collected from four experimental sites from plots containing cereals (barley and/or wheat) in late 2022 and re-sampled 12 months later. Locations include 2 experimental sites at Trangie (one characterised as ‘grey soil’ and one ‘red soil’), one at Spring Ridge, and one at Narrabri. Main tillers from 25 stubble butts per plot were sterilised and cultured as described above, except that stem pieces were assessed in 5 cm increments. Note the average Fp incidence for each sampling time have been reported here without statistical analysis (Figure 4).
Figure 4. Preliminary data of the incidence of Fp recovery (Fp (n) being number of tillers producing Fp colonies) at 5 cm increments along the stubble length (stubble height, cm) at four different Northern Farming Systems experimental sites in northern NSW.
These preliminary data appear to have a similar pattern occurring at all four sites over the 12 months: there has been a general reduction in pathogen recovery in the crown (0 cm), maintenance of Fp incidence in the lower stem (5 cm) and then an increase in Fp incidence from approximately 10 cm and above (Figure 4). This reflects the rapid displacement of Fp from the crown by other soil microbes in the first year, but also reinforces how persistent Fp can be in the upper parts of retained cereal stubble. These results are in line with findings from randomised and replicated field experiments conducted on inoculated durum wheat stubble from 2019 to 2020 (Petronaitis et al., 2022), suggesting that the pattern of saprotrophic colonisation is repeatable across different cereal crops, seasons and environments. Extensive stubble survey work conducted under a FCR co-investment (GRDC and NSW DPI, project code DPI2207-004RTX) will be used to further explore the frequency and extent of saprotrophic colonisation of stubble by Fusarium species in grower paddocks. This will help to better understand what factors may promote saprotrophic colonisation of retained cereal stubble by Fp in the farming system.
Conclusions
Preventing infection of cereal plants by Fp offers the greatest protection from FCR. For now, partial resistance to FCR offers benefits like slowing infection and reducing yield loss. Even the more resistant winter cereals such as oat can still get infected by Fp but can be asymptomatic (i.e., do not always express FCR) so Fp may go undetected in these crops. Inoculum may then accumulate after harvest once plant defence mechanisms are no longer active. Preliminary field results are showing that Fp incidence increases within the stems of stubble across a range of environments and crop types in the first year after harvest. More work is needed to understand what drives saprotrophic colonisation and the implications for FCR risk in future seasons.
References
Knight NL, Sutherland MW (2017) Assessment of Fusarium pseudograminearum and F. culmorum biomass in seedlings of potential host cereal species. Plant Disease 101:2116-2122.
Nelson KE, Burgess LW (1995) Effect of rotation with barley and oats on crown rot of wheat in the northern wheat belt of New South Wales. Australian Journal of Experimental Agriculture 35:765-770.
Petronaitis T, Forknall C, Simpfendorfer S, Backhouse D and Flavel R (2022) Harvest height implications for Fusarium crown rot management. GRDC Update, online, 2 March 2022.
Petronaitis T, Forknall C, Simpfendorfer S, Backhouse D (2020) Stubble Olympics: the cereal pathogen 10cm sprint. GRDC Update, Goondiwindi, March 2020.
Summerell BA and Burgess LW (1988) Stubble management practices and the survival of Fusarium graminearum Group 1 in wheat stubble residues. Australasian Plant Pathology, 17:88-93.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. Thanks also to the Grains Agronomy and Pathology Partnership (GRDC and NSW DPI) which co-funded part of this research through Dr Petronaitis’ GAPP PhD scholarship.
Thanks also to Dr Cassy Percy for providing germplasm for the glasshouse experiment, and Branko Duric for providing stubble from the Northern Farming Systems project. Additional technical support provided by Nicole Dunn, Barbara Jones, Alana Roe, and Caroline Stewart is gratefully acknowledged.
Contact details
Toni Petronaitis
University of New England
Armidale, NSW 2351
Ph. 0424 209 816
Email: tpetrona@une.edu.au
Twitter: @ToniPetronaitis
Date published
February 2024
Varieties displaying this symbol are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: DPI2207-004RTX, DAQ2007-002RTX,
Was this page helpful?
YOUR FEEDBACK