Soil and plant tissue testing for diagnosing herbicide carryover risk - an update
Soil and plant tissue testing for diagnosing herbicide carryover risk - an update
Take home messages
- Soil and plant tissue analysis for herbicide residues is not a replacement for using herbicides according to label requirements, but can provide additional context for decision making.
- Several soil-crop-herbicide toxicity thresholds are now available to help interpret soil tests, noting there will always be uncertainty in interpreting potential crop effects due to in-paddock variation, environmental conditions, and cultivar-specific tolerances.
- Leaf tissue testing is being developed as an additional tool to help diagnose herbicide residue damage and correlations with soil residues.
- Ongoing work will increase the interpretability of soil and plant tissue testing for herbicide residues. Growers and agronomists can help by providing feedback on the usefulness of any residue testing they undertake.
Background
The impact of herbicide residue carryover on crop yields, profitability and sustainability of the Australian grains industry is uncertain. Recent work (GRDC and Soil CRC co-funded; Rose et al. 2022) estimated that 20–30% of paddocks across Australia contain soilborne herbicide residues that constrain crop selection, while around 10% of paddocks have herbicide residues that could result in crop damage and economic losses to growers. The primary drivers of herbicide breakdown are qualitatively well known; however, simple decision support tools to predict the level of herbicide remaining in soil over time, and the risk to rotation crops, are not currently available.
Here we describe an update to our previous 2022 paper (https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2022/02/soil-and-plant-tissue-testing-for-herbicide-residues-how-can-it-help) showing how soil and plant tissue testing can be used to help assess and diagnose herbicide carryover risk and damage.
Soil testing
Recap of key sampling/analysis recommendations
In our previous GRDC research update paper, we provided recommendations for soil sampling and submission for herbicide residue testing. A summary of the main considerations is presented here but for full details, please refer to the 2022 Update paper online (https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2022/02/soil-and-plant-tissue-testing-for-herbicide-residues-how-can-it-help).
Where and how many samples?
- Herbicide concentrations in surface soil (0–10cm) can vary by a factor of 10 across a paddock.
- Sampling should target at least two areas within a paddock of known contrast in crop performance (for example, areas of poor versus vigorous crop growth) or landscape/soil contrast (for example, dunes versus swales; heavier-textured soils versus lighter-textured soils).
- Within each target area, take a minimum of three soil cores and composite these to obtain a representative soil sample for that area.
- Note that herbicide concentration can differ substantially between the crop row and inter-rows, so consider likely seeding location of upcoming crop.
Sampling depth
- Residues are usually highest at 0–10cm depth and this section of the profile should be sampled as the primary interest, as this is where the new crop is germinating.
- Where there is reason for concern about possible residues at depth (for example, soil inversion, texture contrast soils), additional sampling can target these soil sections.
When to sample
- Sampling should consider time before sowing (residues may further degrade in warm, wet conditions) and allow for a 3–6-week turnaround for analysis and decision-making.
Sample handling
- Samples (~500g) should be refrigerated or frozen promptly to prevent microbial breakdown of any herbicides present. Use cool transport (for example, with ice bricks) to maintain sample integrity.
Laboratory analysis
- Choose labs with NATA accreditation (https://nata.com.au/find-organisation/) for certified herbicide residue testing methods (for example, search ‘herbicide’ in Keywords). Discuss with laboratory whether a single residue (that is, one active, less expensive) or multi-residue analysis (that is, multiple herbicide classes, more expensive) is required.
- Ensure detection levels are appropriate for potential toxicity thresholds, especially for sensitive herbicides like sulfonylureas or imidazolinones (see Table 1 below for suggested ‘limits of reporting’ – LORs).
Interpreting laboratory results
During the 2022 GRDC update (Rose et al. 2022), we presented the rationale for interpreting soil test results for herbicide residues. A commercial laboratory will report back the concentration of one or more herbicide actives in the samples submitted, usually in mg herbicide per kg dry soil (mg/kg). If a multi-residue analysis has been conducted (sometimes up to 30 or more active ingredients), many herbicides will often be reported as <LOR (less than the Limit Of Reporting) or <LOQ (less than the Limit Of Quantification). These indicate that the herbicide is not present at levels above the testing limits, but trace levels may still be present below these limits.
Decision making becomes somewhat straightforward where the laboratory report indicates residues are not present. However, where residues are present, they need to be compared against an established ‘effective dose’ (ED) damage threshold (mg/kg, or equivalent units) that has been generated in a similar soil type. Where available, effective dose (ED) damage thresholds are usually presented as ED10, ED20 or ED50, equivalent to the concentration causing 10, 20 or 50% biomass reduction. Note that ED values can vary greatly depending on soil type, so these should be used as a guide only and not a definitive guarantee of the level of damage to be expected.
For most herbicides, there is little available information to guide this decision-making process. Where data have been generated, it is generally not present in a format readily available and easily accessible to agronomists. Table 1 is a summary of the number of known data sets x herbicide x crop and the confidence level of these data. The current project, DPI2306-013RTX, intends to develop some additional information for selected high priority herbicide-crop combinations. More information about specific ED values is available on request.
Table 1: Soil toxicity threshold values available for selected Australian grains herbicide-winter crop combinations at the seedling stage (2–6 weeks after sowing). Blank cells = no data available; L = low confidence (≤3 values available); M = moderate confidence (4–7 values available); H = high confidence (≥8 values available). Wht = Wheat, Bar = Barley, Can = Canola, Lup = Lupin, Pea = Field Pea, Len = Lentil and Chick = Chickpea. Data have been compiled from Rose et al. (2022), GRDC project US00084 and Soil CRC project 4.2.001.
| Herbicide Group | Active | Crop | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wht | Bar | Oat | Can | Lup | Pea | Len | Chick | ||
| 2 | Imazapic | M | M | M | |||||
| Imazapyr | L | L | L | L | L | L | L | L | |
| Imazamox | |||||||||
| Imazethapyr | |||||||||
| Flumetsulam | |||||||||
| Chlorsulfuron | M | L | |||||||
| Triasulfuron | L | L | M | L | M | ||||
| Metsulfuron-methyl | L | L | |||||||
| 3 | Trifluralin | M | H | L | |||||
| Propyzamide | |||||||||
| 4 | 2,4-D | L | L | ||||||
| MCPA | |||||||||
| Dicamba | |||||||||
| Aminopyralid | |||||||||
| Picloram | |||||||||
| Fluroxypyr | |||||||||
| Triclopyr | L | ||||||||
| Clopyralid | L | L | L | L | L | L | L | L | |
| 5 | Simazine | L | L | M | M | L | L | ||
| Atrazine | L | M | H | M | L | L | L | ||
| Terbuthylazine | |||||||||
| Metribuzin | L | ||||||||
| Diuron | H | M | L | L | L | L | L | M | |
| 12 | Diflufenican | ||||||||
| 13 | Bixlozone | ||||||||
| 14 | Fomesafen | L | |||||||
| Saflufenacil | |||||||||
| Trifludimoxazin | |||||||||
| Flumioxazin | |||||||||
| 15 | Pyroxasulfone | L | L | L | L | L | L | L | L |
| Prosulfocarb | |||||||||
| Tri-allate | |||||||||
| Metazachlor | |||||||||
| s-metolachlor | |||||||||
| 23 | Carbetamide | ||||||||
| 27 | Pyrasulfotole | ||||||||
| Topramezone | |||||||||
| Bicyclopyrone | |||||||||
| Mesotrione | |||||||||
| Isoxaflutole | |||||||||
| 30 | Cinmethylin | ||||||||
| 32 | Aclonifen | ||||||||
| O | Napropamide | ||||||||
Leaf tissue testing
Crop leaf tissue analysis in the future may be a useful diagnostic tool where herbicide carryover damage is suspected but cannot be confirmed by visual symptoms and spray records. Leaf tissue testing for herbicide damage is still in its infancy and presently may not provide conclusive answers for all herbicides, but as this area of technology develops and improves, it will provide another line of evidence to support or rule out the suspected cause for poor crop growth.
Current sampling/analysis recommendations
If herbicide damage is suspected, the above ground biomass of 3–10 plants should be sampled from an area of poor growth and a similar number from an area of better growth within the same paddock. This is a minimum, and more areas can be considered for sampling if better spatial diagnosis is required. Biomass should be cut near to ground level, taking care to avoid contamination with soil. Plants from the same area should be bagged together (that is, bulked) and stored in a cool (but not frozen) environment until samples can be dispatched to the laboratory for analysis, ideally within 24 hours of sampling. The fresh weight of the samples should also be recorded for future reference, to gauge the amount of damage in the areas of poor crop growth relative to the areas of healthily/vigorous growth. Samples should be express couriered to the analytical laboratory with ice bricks in cooler bags or a styrofoam box to keep samples cool during transport. Note that these are recommendations based on our current understanding but may be modified or improved upon in future as more research is conducted.
Interpreting laboratory results
As with soil samples, results will be reported back from the laboratory as herbicide concentrations in biomass sample submitted, usually in mg herbicide per kg dry biomass (mg/kg). At this stage, there is very little information available to help interpret ‘what the numbers mean’ for leaf tissue testing, aside from presence/absence of certain herbicides, helping to rule out potential causes for poor growth. However, recent research in GRDC project US00084 generated leaf tissue toxicity thresholds for diuron, simazine and imazapic to wheat grown in different soil types. Leaf tissue thresholds were more consistent across different soil types compared to the respective soil thresholds (Figure 1 shows diuron toxicity thresholds as an example).
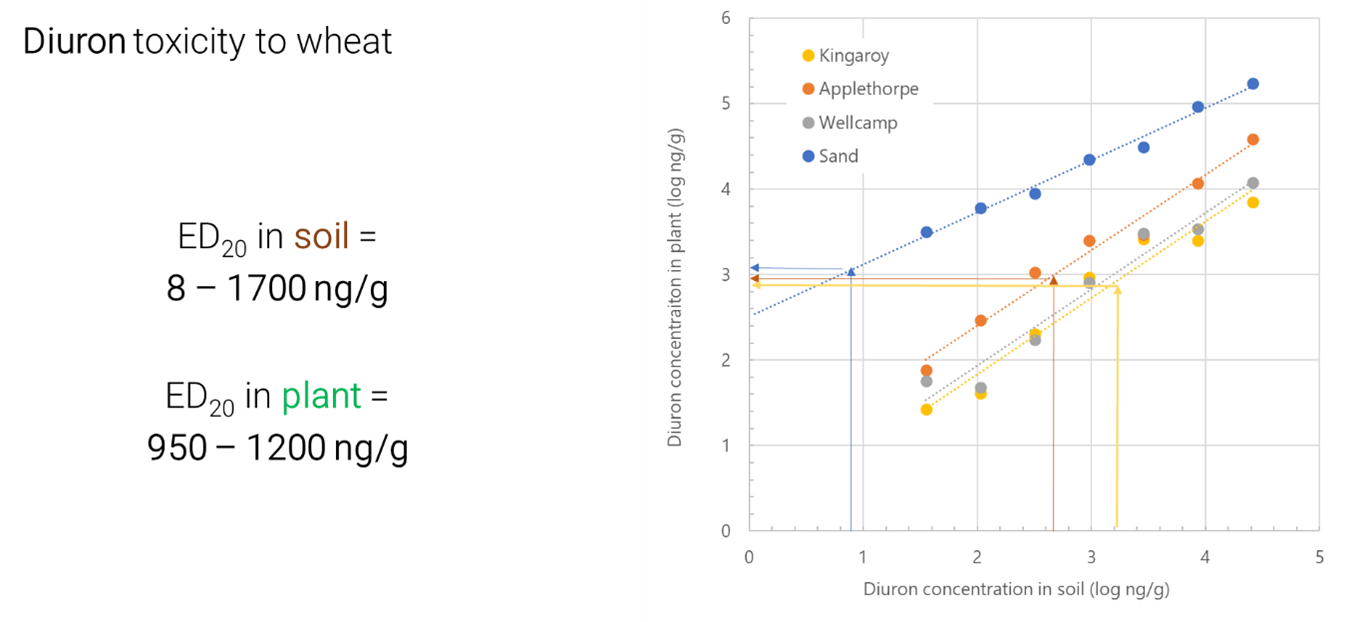
Figure 1. Soil (x-axis) and leaf tissue (y-axis) critical thresholds for diuron toxicity to wheat (ED20, the dose required to reduce wheat shoot biomass by 20% compared to controls). Soils are from Kingaroy (OC = 1.8%, pH = 5.1, Clay = 20%); Applethorpe (OC = 0.6%, pH = 6.1, Clay = 5%); Wellcamp (OC = 1.5%, pH = 7.8, Clay = 39%); Sand (OC = <0.1%, pH = 6.6, Clay = <1%).
This is because herbicide bioavailability differs according to soil type, giving rise to different soil-based thresholds, whereas once the herbicide has been taken up by the crop, the toxicity will be relatively consistent within that species/cultivar. Slight differences may arise in leaf tissue thresholds due to the health of the plant according to other environmental/management conditions. This is similar to critical nutrient thresholds, particularly for soil-immobile nutrients like P, where soil-based critical thresholds are soil type dependent (regulated by P-buffering process represented by the P-buffering index, PBI), but leaf tissue critical thresholds are independent of soil type. This provides proof-of-concept that meaningful leaf tissue thresholds can be generated for priority herbicides.
Relevance of thresholds to field conditions
To date, soil and plant critical thresholds (for example, Table 1) have been generated in controlled, short-term glasshouse experiments on 3–6-week-old crop seedlings. The question arises: how relevant are these thresholds to the growth and yield of crops under field conditions?
Recent experiments in WA to test the effect of soil amelioration (mouldboard plough or spading) on the risk of crop damage by different pre- or post-emergence herbicides provided a platform to address this question for the target herbicide diuron. Field experiments were established through project DAW1901-006RTX, and leaf tissue samples provided to project DPI2306-013RTX for herbicide analysis. Although the focus of the field experiment was not on herbicide residue carryover, the experimental design enabled us to test the hypothesis that:
- leaf tissue testing can diagnose herbicide exposure in crop
- herbicide concentration in leaf tissue at an early growth stage can predict impacts on final crop biomass and yield.
Experiments were established prior to sowing in 2023. Herbicide treatments were applied the day prior to sowing to plots with undisturbed soil (conventional practice) or spaded soil. The target herbicide (for our analysis) diuron was applied to individual plots at label rate or three times label rate for experimental purposes only (that is, 550 or 1650g/ha) (noting that other treatments were also applied but were not the focus of our study). Control plots did not receive any herbicide application. Wheat (Scepter) was sown on 31 May 2023. Injury scores, NDVI and per cent ground cover were recorded on 10 July 2023, at the same time that leaf tissue samples were taken for herbicide analysis at NSW DPI, Wollongbar.
Soil amelioration (undisturbed vs spaded) did not significantly (P>0.05) affect the concentration of diuron in the wheat leaves at 6 weeks after sowing, but higher application rates resulted in significantly higher diuron leaf tissue concentrations (P<0.05) (Table 2). Leaf tissue concentrations of diuron were negatively correlated with early season growth (NDVI, groundcover) and biomass at harvest, but not yield (Figure 2).
Table 2: Herbicide concentration (ng/g in wheat leaf tissue at 8 weeks after sowing and least significant difference (bold, bottom row) estimated by ANOVA model.
| Rate | Herbicide concentration (mg/kg) |
|---|---|
| Nil | <LOQ (0.01) |
| Label rate | 0.58 |
| 3 x Label rate | 2.24 |
| Lsd (P<0.001) | 0.42 |
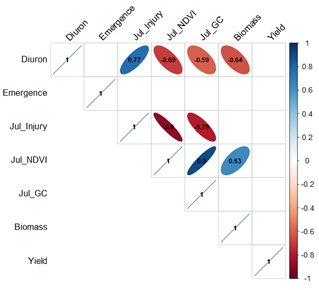
Figure 2. Correlation plots of diuron concentration in leaf tissue (‘Diuron’) against crop growth and yield measures. Only significant (P<0.05) relationships between variables are shown. Blue represents a significant positive relationship and red represents a significant negative relationship; numbers indicate correlation (r2) values.
Importantly, the leaf tissue ED20 thresholds generated in glasshouse studies (0.95–1.20mg/kg) closely matched the ED20 values for ground cover (0.9mg/kg) and NDVI (1.30mg/kg) from the field study (Figure 3). These results provide confidence that glasshouse-derived thresholds will be relevant under field conditions, particularly early season growth. However, early season vegetative suppression may not strongly correlate to final yields, since this will depend on growing conditions throughout the entire season, in particular, available soil moisture post-anthesis. Field validation is still required for other herbicide actives and crop types.
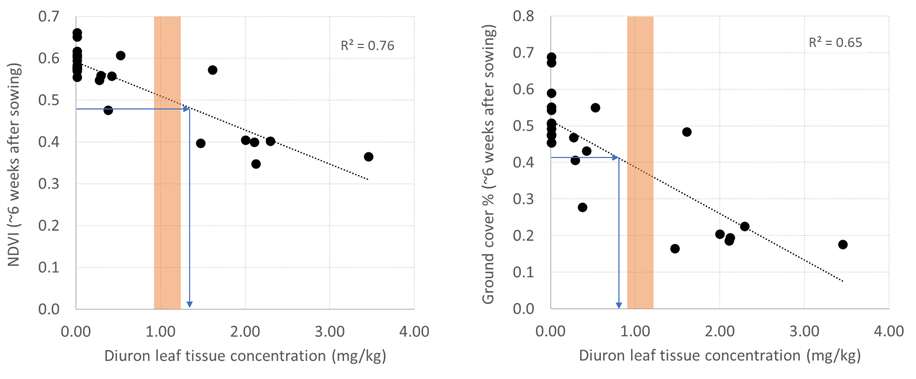
Figure 3. Relationship between diuron leaf tissue concentrations and early wheat growth measured as NDVI (LHS) or ground cover (RHS). Soil and leaf tissue critical thresholds for diuron toxicity to wheat (ED20) are shown by blue arrows for field data, and the orange bar for previous glasshouse data.
Ongoing research
Generating and validating new soil and plant toxicity thresholds
A new GRDC NGN project, ‘Understanding herbicide residues on challenging soil types within the southern region’, will target soil types in SA, including the regions around Wirrulla, Wangary, Jamestown, Balaklava, Warooka and Bowhill, to develop dose response curves (toxicity thresholds) for sensitive crop-herbicide combinations. This will add to existing work in GRDC project DPI2306-013RTX that is also developing additional soil-crop-herbicide toxicity thresholds to help interpret soil and leaf tissue tests and support spatial risk assessments for herbicide residues as outlined below.
Spatial modelling of herbicide persistence and carryover risk
Current work in DPI2306-013RTX aims to develop a geospatial analytics tool (for example, software) that can assist in predicting herbicide residue carryover and crop risk in different soil types and cropping environments. Work in 2024 involved establishment of four core sites (Table 3) in south-east Qld, southern NSW, Eyre Peninsula SA and northern WA wheatbelt to measure persistence of four or more herbicides, at two rates, on two soil types per site. At the south-east Qld site, treatments were also applied with and without supplementary irrigation. These protocols will be repeated at additional sites selected in 2025.
Table 3: Target herbicides applied in 2024 were selected to cover a diversity of physical and chemical properties.
| Active | MOA | SE Qld | Sth NSW | EP SA | Northern WA |
|---|---|---|---|---|---|
| metsulfuron | 2 | X | X | X | X |
| imazapic + imazapyr | 2 | X | X | X | |
| imazapic | 2 | X | |||
| clopyralid | 4 | X | X | X | |
| terbuthylazine | 5 | X | X | X | X |
| diuron | 5 | X | X | ||
| diflufenican | 12 | X | |||
| flumioxazin | 14 | X | X | X | |
| fomesafen | 14 | X | X | ||
| pyroxasulfone (one rate only) | 15 | X | X | X |
Field plots with ‘carryover residues’ will be planted with a range of rotation crops in 2025 to assess plant-back damage and provide validation of soil and leaf tissue testing concepts and models. At each site, multiple soil residue samples will be taken, at two depths for at least 18 months post-application, along with in-crop plant samples from varieties planted in year one of the trial and rotational crops to be planted in year two. Additional crop measurements include germination counts, NDVI, plant biomass, nodulation of pulses and yield. In addition, a network of satellite paddock monitoring sites will provide additional environmental and soil type variation to validate spatial modelling approaches. At the completion of the field data collection and sample analysis, the project intends to develop a computer model to assist users in predicting the range of herbicide persistence and risk to following crops that could be expected across a range of growing regions and soils in the Australian grains industry.
Conclusion
Soil and plant tissue testing for herbicide residues is technically feasible and sensitive for low concentrations, but historically, it has been difficult to interpret the test results. Work over the last five years has developed and consolidated toxicity thresholds that can now be used to help interpret soil tests for certain herbicide-crop combinations. Leaf tissue testing is still in its infancy but is showing promise for helping to diagnose the cause and level of possible herbicide damage. Ongoing work will further refine and validate these tools for future management of herbicide carryover.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support. This work has also been supported by the Cooperative Research Centre for High Performance Soils whose activities are funded by the Australian Government's Cooperative Research Centre Program, through project 4.2.001. Thanks to Brad Keen, Kelvin Spann, Scott Petty, Ken Lisha, Alex Watson-Deal, Annie Rutledge and Jesse Muller for input and technical support.
References
Rose MT, Zhang P, Rose TJ, Scanlan CA, McGrath G, Van Zwieten L (2022) Herbicide residues in Australian grain cropping soils at sowing and their relevance to crop growth. Science of the Total Environment 833, 155105.
Herbicide behaviour (https://grdc.com.au/resources-and-publications/resources/herbicide-behaviour)
Residue watch: how do I know if herbicide residues are breaking down before sowing (https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2021/07/residue-watch-how-do-i-know-if-herbicide-residues-are-breaking-down-before-sowing)
Soil and plant tissue testing for herbicide residues – how can it help (https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2022/02/soil-and-plant-tissue-testing-for-herbicide-residues-how-can-it-help)
Soil CRC webinars (https://soilcrc.com.au/webinars/)
Contact details
Michael Rose
Southern Cross University
Military Rd, East Lismore NSW 2480
0422 522 774
mick.rose@scu.edu.au
@Mick_T_Rose
GRDC Project Code: DPI2306-013RTX, DAW1901-006RTX, UOS1703-002RTX,
