Insect pests - resistance, virus vectors and lessons from 2014
Author: Garry McDonald and Paul Umina (cesar) | Date: 18 Mar 2015
Take home messages
- The green peach aphid (GPA) and diamondback moth (DBM) were widely reported to PestFacts south-eastern in 2014. An outbreak of Beet western yellows virus (BWYV), transmitted by GPA, was widespread in many areas.
- These pests and others, were initially encouraged by an early, wet and mild autumn which, among other factors, stimulated widespread growth of late summer and autumn weeds, creating a ‘green bridge’.
- GPA and DBM have a high prevalence of resistance to insecticides. Growers should implement resistance management strategies for insecticides.
- Redlegged earth mites (RLEM) have now acquired resistance to both synthetic pyrethroids and organophosphates in some areas of WA but not in south-eastern Australia. Growers experiencing any chemical control failure of RLEM should contact us.
Background
Seasonal climate provides the overwhelming influence on populations (“outbreaks”) of in-field pests. 2014 provides an excellent example of this reality. In the 2014 growing season, much of Victoria experienced an exceptionally early, wet and mild autumn which favoured many groups of pests. In contrast, the harsh conditions that followed in winter (severe frosts) and an abrupt and dry end to spring discouraged other pest groups.
For those of us providing advice on crop pests in south-eastern Australia through the PestFacts Service (South Eastern and South Australia), 2014 was an exceptional year for enquiries and reports. PestFacts south-eastern took 42% more enquiries than in 2013, and 19% more than the previous busiest year (2007). Of the 55 pests reported, the most notable pests were the green peach aphids (Myzus persicae) and the virus it vectors beet western yellows virus (BWYV, syn. Turnip yellows virus) in canola, the common cutworm (Agrotis infusa) in all crops and the diamond back moth (Plutella xylostella) in canola.
PestFacts is a GRDC funded e-mail service provided by cesar (PestFacts south-eastern) and SARDI (PestFacts South Australia) designed to keep growers and farm advisers informed about invertebrate issues, and solutions, as they emerge during the winter growing season. The service has a focus on pests of broad-acre grain crops. Throughout the season, PestFacts subscribers provide us with pest reports and field observations on the appearance and distribution of invertebrate pests across south-eastern Australia. We use this information to regularly produce the PestFacts newsletter and provide advice and recommendations to hundreds of individuals, organisations and businesses working with broad-acre crops and pastures.
In this paper, we provide a brief overview of the major pests of 2014, the lessons for their management, the implications (if any) for 2015, and an update on research undertaken on insecticide resistance in GPA, DBM and RLEM.
Season overview 2014
Pest reports
The PestFacts south-eastern service has been in operation for nine years and from this we have built a profile of the seasonal and regional distribution of pests (see Pestfacts Map cesar Australia website) based on reports to us from growers, advisers and researchers. From this data, we can deduce that more than eight pests were reported at least twice as commonly in 2014 than in other years (Figure 1). This is not to say that other pests such as redlegged earth mite, blue oat mite, and lucerne flea were not a problem in 2014, because they were in many cases, but just that there was not exceptional reporting of these pests.
For example, the 32 reports of common cutworms in 2014 represented 78% of all cutworm reports since 2006. Similarly, the 26 reports of GPA and 15 reports of DBM were 67% and 60% of all reports, respectively. It is not unusual for some pests to dominate in some years, but the very strong prevalence of these pests was exceptional.
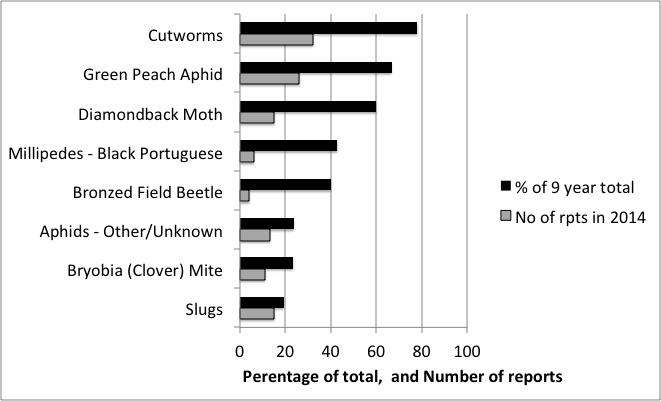
Figure 1. Pests occurring in 2014 that were reported at least twice as commonly as that expected from the average annual reporting of pests.
Seasonal conditions
In summer/autumn 2014, conditions were excellent for plant growth in much of south-eastern Australia. In February 2014, heavy rain fell across south-western NSW, parts of the Victorian Mallee and South Australia. Again in April, heavy rains fell across the cropping zone in NSW, SA and the northern reaches of Victoria. Mild temperatures persisted through May into early June. These conditions were perfect for many pests that exploit summer weeds to sustain and build their populations prior to the cropping season.
Frosts were extreme in July and August and spring was exceptionally dry and hot. Figure 2 summarises these conditions for Swan Hill as one such example.
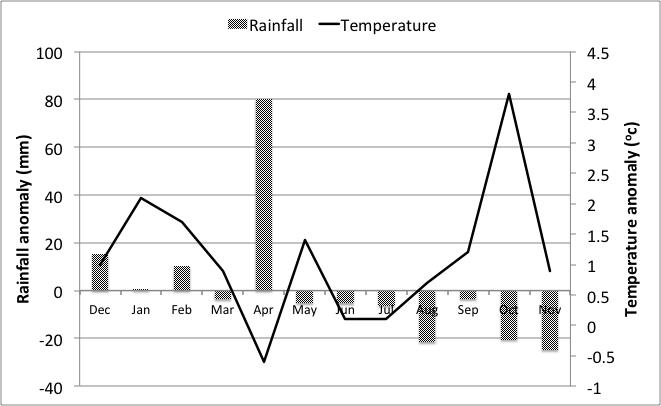
Figure 2. Temperature and rainfall anomalies for Swan Hill, Dec 2013 - Nov 2014.
Green peach aphid and beet western yellows virus
Widespread infestations of green peach aphids (GPA) during autumn and winter of 2014 contributed to an outbreak of beet western yellows virus (BWYV) in southern Australia. At the height of the outbreak high numbers of GPA (>5 per leaf on every plant) were being recorded in some areas. Canola crops across the lower and mid north regions of South Australia, the Eyre Peninsula, western Victoria and some parts of NSW were severely affected by the virus.
GPA, the principal vector of BWYV, has an oval shaped body, and may be pale yellow-green, orange or pink in colour. The adults are approximately 3mm long; winged adults have a black patch on the abdomen. Wingless forms are uniform in color. Their presence on the underside of leaves is also diagnostic.
BWYV
Symptoms of the virus may initially resemble nutrient deficiencies, herbicide damage or other disorders. Leaves may turn red, purple or yellow, starting from the lower leaves. Other symptoms may include leaf mottling, leaves become thickened and cupping inwards, and premature bolting. Symptoms are milder and stunting is lacking with late infections, which generally result in a minimum effect on yield.
BWYV is not seed-borne and survives from one growing season to the next in over-summering canola, broad-leafed weed species, and perennial legume pastures. BWYV is spread by several aphid species that colonise canola, particularly GPA.
GPA and insecticide resistance
Together with CSIRO, cesar have been mapping the extent of insecticide resistance in GPA across Australia for the past few years. In response to the BWYV outbreak in 2014, approximately 50 populations were tested in south-eastern Australia. Resistance testing of populations sampled from the different regions revealed widespread resistance to the three chemical groups tested. Following DNA tests, aphids from all populations were found to be resistant to synthetic pyrethroids, specifically bifenthrin and alpha-cypermethrin, and to carbamates, specifically pirimicarb. The use of these insecticides is not expected to provide control against these GPA populations in most field situations. The mechanism of resistance to pyrethroids is also likely to render the anti-feeding properties of these products ineffective.
The story for organophosphates, such as dimethoate and chlorpyrifos, is more complex. The amplified carboxyl-esterase mechanism leads to organophosphate resistance in GPA. This mechanism is unusual because it is regulated by DNA methylation, and can be ‘switched on’ in response to pesticide exposure. As a result, aphid populations can quickly adapt to survive organophosphates, even though they may have recently been effective. Following DNA tests, all aphid populations tested were found to contain resistance at the esterase genes. Aphids from these populations are expected to have a moderate level of resistance (5-20-fold) to organophosphates.
The field efficacy of organophosphates is currently unpredictable. Some control may be achieved against populations found to be ‘resistant’, although the use of organophosphate insecticides is risky and may not be effective (particularly if the population has been exposed to insecticides already in the season). When faced with GPA populations with known resistance to organophosphates, growers are advised to spray test strips within paddocks to determine field efficacy.
There is no confirmed resistance to neonicotinoids in GPA, however widespread resistance is known overseas and there is some anecdotal evidence that resistance may be evolving in Australia. Formal testing for neonicotinoid resistance in a number of GPA populations from across Australia will be undertaken in 2015.
2014 and 2015
The severity of the 2014 BWYV outbreak is probably due to a combination of the following factors:
- Summer rainfall which resulted in a ‘green bridge’ of weed hosts of BWYV and aphids.
- The early start to the season and early sowing.
- Very mild autumn conditions which contributed to early (and extended) levels of aphid activity through till late June.
- Crop management practices.
- The prevalence of insecticide resistance in GPA.
- The low proportion of canola seed treated with imidacloprid in some areas.
Preparing for 2015, there are a number of approaches that should reduce the risk of aphids:
- Where practical, reducing the green bridge in and around properties is valuable. Ideally, this should be an area-wide management approach. An individual farmer who controls weeds on his property over summer and early autumn will gain some benefit, but if neighbours do not undertake similar strategies, GPA can still fly around and infest these crops in autumn and winter.
- Paying attention to weather conditions leading into this year’s growing season will provide growers with a good indication of the likely risk of BWYV. This can be achieved through early monitoring for aphids on brassica weeds, volunteer canola and alternate plants before crops are sown. Avoiding early sowing is also likely to reduce the risk of aphid colonisation and build-up in emerging crops.
- Sowing canola crops into paddocks with standing stubble may also reduce the risk of aphids colonising emerging crops.
- The use of seed treatments containing a neonicotinoid insecticide can provide protection of canola seedlings from aphid attack during the early growth stages.
- Recognising the widespread resistance in GPA to pyrethroids, organophosphates and carbamates, using Transform® when aphid numbers have reached threshold levels should be considered. This product should be used as part of a broader resistance management strategy. A recently completed resistance management strategy for GPA (IPM guidelines for grains website) provides useful guidance.
Redlegged earth mites
Insecticide resistance in RLEM
Insecticide resistance was first identified in Western Australia (WA) in 2006. Resistance was confirmed to synthetic pyrethroids (SPs – Group 3A), including bifenthrin and alpha-cypermethrin. There are now more than 30 properties affected in that State, but research indicates resistant populations are likely to be widespread across the WA grain belt and may move into parts of eastern Australia.
Every season RLEM cause millions of dollars worth of damage to crops and pastures and insecticides are the main control option. But continued use of these products puts selection pressure on mites to develop resistance, leading to control failures and crop losses.
Our research indicates SP-resistant RLEM are up to 240,000 times more resistant to some SP insecticides than susceptible RLEM and this resistance is genetic; surviving through several generations.
Until 2014, resistance in RLEM was confined to SPs and there was no evidence of cross-resistance to other insecticide groups. However, in spring 2014, RLEM were identified surviving field applications of organophosphates (OPs – Group 1B) at a single property in WA. These mites were subsequently tested by DAFWA entomologists and by cesar. Unfortunately, these mites were shown to have developed resistance to OPs, such as dimethoate and omethoate.
Screening of more than 50 field populations across NSW, Victoria and SA in 2014 found no evidence of insecticide resistance in RLEM. That being said, growers experiencing any chemical control failure involving mites should contact us immediately. A free diagnostic testing service funded by GRDC is available to growers in 2015.
Predicting the hatch of first generation RLEM
The main hatch event of RLEM occurs in late autumn when conditions of rainfall and temperature are suitable. The timing of egg hatch in relation to that of crop emergence will influence the damage potential of the mites, and the need to monitor and perhaps control. We have now published a model that uses both rainfall and daily temperatures to estimate the date of egg hatch. The research also concluded that, in south-eastern Australia, ten days of average daily temperatures of 16°C, rather than 20.5°C as previously thought, are required to trigger egg hatch in autumn or early winter. The model will be used by the PestFacts service to assist growers and provide greater certainty around periods when paddocks are at risk of mite damage.
Diamondback moth
DBM caterpillars are pale yellowish green and tapered at each end of their body, and grow to about 12 mm long. The moths are about 10 mm long and grey-brown in colour with a characteristic whitish strip of uneven width down the back, which resembles diamond patterns. The moths are active at dusk and throughout the night, but usually do not fly far within a crop. However, outside the crop, they can migrate long distances (potentially hundreds of kilometers) on prevailing winds, especially when their host plant has died.
DBM is widely distributed across Australia, but are most common in southern states during spring and summer.
2014 outbreak
From July to October 2014, the number of DBM infestations in canola across northern Victoria (particularly the Wimmera and Mallee) appears to have been greater than in most recent years. Although the populations were large, many remained below or at threshold (100 per 10 sweeps on post-flowering crops). Typically, damage varied from limited leaf and pod grazing to areas experiencing economic damage.
The explanation for the exceptionally large winter and spring populations of DBM may be revealed through a PhD study currently being undertaken by Kym Perry (SARDI), funded through the University of Adelaide and GRDC. Kym found DBM larvae during March and April 2014 on wild Brassicaceous summer (weed) hosts, such as Lincoln weed (Diplotaxis tenufolia), dog weed (Diplotaxis muralis) and sea rocket (Cakile maritima) in the canola region of western Eyre Peninsula SA. This suggests that these and other summer-growing brassica weeds might play a key role in maintaining DBM populations in years with wet autumns, and allowing early invasion of canola crops. These results likely reflect the favourable early season weather conditions across southeastern Australia, including February and April rainfall that promoted the growth of Brassica hosts, and above-average temperatures in May that promoted population development and flight activity. However the extent to which early crop colonisation contributed to spring population levels is still unclear. The Zoopthora fungal infection often found in winter will also limit DBM build-up when wet weather conditions persist.
Insecticide resistance
As with several other important grain pests, DBM has a high propensity to develop resistance to chemicals. There is resistance to various insecticides within Australian DBM populations (Table 1). Once sweep-netting indicates DBM larval densities are at the spray threshold, a quick response with a spray can give adequate control of larvae and reduce yield losses. Under particularly heavy or persistent infestations, two spray applications (five to seven days apart) may be necessary. To reduce the risk of resistance developing to these newer insecticides, spray for DBM only when thresholds are exceeded and alternate between insecticide groups from one season to the next, assuming one or two sprays per season.
Table 1. Insecticide resistance in diamondback moth.
| Insecticide Group | Known resistance | Status |
|---|---|---|
| Group 1A (Methomyl)* | Yes | ? |
| Group 1B (Organophosphates) | Yes | Widespread |
| Group 3A (Synthetic Pyrethroids) | Yes | Widespread |
| Group 5 (Success Neo®) | No (or very low) | Limited |
| Group 6 (Affirm®)** | Yes | Common |
| Group 11 (Dipel) | No | ? |
| Group 28 (Coragen®)*** | Yes | Common |
Source: G. Baker and K. Powis (SARDI)
* Methomyl has not been tested because (1) it only has WA registration, (2) it only has low-moderate efficacy even on Waite Susceptible strain, (3) old DBM literature indicates that there’s OP-Carbamate cross-Resistance, and we know we have widespread OP Resistance
** Affirm R recorded so far in canola is at relatively low levels, not yet having observable effects on field efficacy.
*** Note that there are no Group 28’s registered in canola (but there is in vegetables).
Conclusion
The 2014 winter growing season commenced with unseasonably wet and mild conditions in many cropping regions of south-eastern Australia. These conditions certainly suited early crop establishment, but also favoured a number of pests even before crops were planted.
As a direct result, unusually severe infestations of many pests soon became apparent. The growth of summer weeds was the means by which many pest populations started the 2014 season in such large numbers.
The events of 2014 are unlikely to be repeated in 2015 unless similar late summer/autumn conditions occur and the’ green bridge’ is not appropriately managed. Last season also highlighted the need to manage pesticide usage carefully to minimise the growing threat of resistance.
Acknowledgements
This paper has drawn on data and experience from a number of specialists, for which we are grateful.
- Jenny Davidson, Greg Baker, Bill Kimber, Helen de Graaf, Kym Perry, Ken Henry (SARDI) and Mohammad Aftab(Vic DEPI): Survey of management practices affecting BWYV in canola 2014 and the BWYV / GPA epidemiology story.
- Greg Baker and Kym Perry on DBM ecology and pesticide resistance and for an earlier version of the 2014 climate analysis.
- Siobhan de Little, Andrew Weeks and Anthony van Rooyen (cesar) and Owain Edwards (CSIRO): GPA resistance.
- Peter Mangano and Alan Lord (DAFWA): RLEM resistance
Resources/further reading
Coutts, B.A., Hawkes, J.R. and Jones, R.A.C. (2006) Occurrence of Beet western yellows virus and its aphid vectors in over-summering broad-leafed weeds and volunteer crop plants in the grainbelt region of south-western Australia. Australian Journal of Agricultural Research 57:975-982.
McDonald, G., Umina, P.A., MacFadyen, S., Mangano, P., and Hoffmann, A.A. (2014). Predicting the timing of first generation egg hatch for the pest redlegged earth mite Halotydeus destructor (Acari: Penthaleidae). Experimental and Applied Acarology DOI 10.11007/s10493-014-9876-x
UminaP.A., Edwards, O., Carson, P., van Rooyen, A. and Anderson, A. (2014). High levels of resistance to carbamate and pyrethroid chemicals widespread in Australian Myzus persicae (Hemiptera: Aphididae) populations. Journal of Economic Entomology 107: 1626- 1638.
Resistance Management Strategy for the Green Peach Aphid in Australia Grains
Contact details
Paul Umina
03 9349 4723
pumina@cesaraustralia.com
Garry McDonald
0419 521 238
gmcdonald@cesaraustralia.com
Was this page helpful?
YOUR FEEDBACK
