NSW fungicide resistance update - status of cereal pathogens
NSW fungicide resistance update - status of cereal pathogens
Take home messages
- The G143A mutation, which confers resistance to Group 11 (Qol, strobilurins) fungicides such as azoxystrobin, was not detected in any of the 94 Septoria tritici blotch (STB) samples submitted from NSW between 2022–24
- The Cyp51 G1 mutation (formerly identified as Cyp51 Isoform 11), which confers reduced sensitivity to Group 3 fungicides (DMI, triazoles) was present in most STB samples from NSW across seasons
- In 2023, the first instances of reduced sensitivity (RS) and resistance to Group 3 fungicides (DMI, triazoles) and Group 7 (SDHI) were detected in net-form of net-blotch (NFNB) and Group 3 (DMI, triazoles) RS in spot-form of net-blotch (SFNB) in NSW. Multiple mutations were recorded
- There is no recorded fungicide resistance in NSW barley loose smut samples to active ingredients tebuconazole (Group 3, DMI, triazoles), fluxapyroxad (Group 7, SDHI) and azoxystrobin Group 11 (Qol, strobilurin)
- High frequency of Group 3 (DMI, triazoles) ‘gateway’ mutation F136 and low to moderate Group 11 (QoI,strobilurin) resistance (G143A) in 19 wheat powdery mildew samples from NSW and southern Qld tested in 2022
- Specific wheat leaf rust, barley leaf rust and barley grass stripe rust pathotypes have been shown in controlled environment testing to have fungicide resistance. These pathotypes have not yet been definitively proven to be associated with in-field fungicide failure
- It is important to submit leaf samples to CCDM and rust samples to The University of Sydney for fungicide insensitivity screening. Local pathologists can assist with sample submission
- Integrated disease management (IDM) options are important to prolong the life of registered fungicide compounds.
Introduction
Fungicide resistance poses a significant challenge to broadacre crop production in Australia, threatening the effectiveness of vital fungicide actives used to manage crop diseases. The inherent genetic diversity within pathogen populations provides a basis for naturally occurring genetic changes, known as mutations, within individuals which can lead to increased risk of resistance development. These resistant individuals are then selected for and become the dominant pathogen population by repeated fungicide exposure, potentially resulting in poorer disease control, reduced yields, higher production costs, and economic losses. To address this issue, implementing Integrated Disease Management (IDM) practices is essential to maintain profitability. IDM combines non-chemical practices with targeted fungicide use, reducing reliance on a single management strategy and thereby extending the effective lifespan of fungicide compounds.
This paper outlines changes in fungicide resistance within pathogen populations and dynamics, emphasising the importance of stewardship-based approaches for sustainable disease management. It serves to consolidate the latest updates on fungicide resistance relevant to NSW in a single document, drawing on studies and extension material from NSW Department of Primary Industries and Regional Development (NSW DPIRD), Centre for Crop and Disease Management (CCDM), University of Sydney, FAR Australia, and the Australian Fungicide Resistance Network (AFREN).
Terminology
Sensitive
Fungi are considered sensitive when they are killed by a fungicide at recommended label rates (AFREN, 2024)
Reduced sensitivity (RS)
Fungi are considered as having reduced sensitivity to a fungicide when an application does not work optimally but does not completely fail. In most cases, this would be related to small reductions in product performance, which may not be noticeable at the field level. In some cases, growers may find that they need to apply the maximum label rate of the fungicide to obtain the previously experienced level of control. Reduced sensitivity needs to be confirmed through specialised laboratory testing (AFREN, 2024)
Resistant (R)
Resistance occurs when the fungicide fails to provide an acceptable level of control of the target pathogen in the field at the maximum label rate. Resistance must be confirmed with laboratory testing, and be clearly linked with an unacceptable loss of disease control when using the fungicide correctly in the field (AFREN, 2024)
Lab detection (LD)
Measurable differences in the sensitivity to the fungicide when the fungus is tested in vitro using tests recognised by the scientific community and/or detection of known or novel molecular mechanisms (e.g. genetic mutation, changes in target gene expression, etc.) of a fungal isolate. These changes can often be detected in the laboratory before any loss of fungicide efficacy is detected in the field. Lab detections are used to confirm reports of field resistance or reduced sensitivity, or to indicate the potential for resistance or reduced sensitivity to develop (AFREN, 2024)
Overview
In collaboration with research providers, agribusinesses and growers, NSW DPIRD have been submitting priority pathogen samples from the NSW cropping region to CCDM at Curtin University in Western Australia, for fungicide resistance testing. In total 186 samples which included Septoria tritici blotch (Zymoseptoria tritici),net-form of net-blotch (Pyrenophora teres f. teres), spot-form of net-blotch (Pyrenophora teres f. maculata), barley loose smut (Ustilago nuda) and wheat powdery mildew (Blumeria graminis f. sp. tritici) were sent to CCDM for testing from the 2022 to 2024 seasons from across NSW and southern Queensland (Figure 1). Testing results and status of each pathogen across the three main fungicide groups is summarised in Table 1.
The data can also be accessed via the CCDM Pesticide Resistance Integrated Mapping (PRIM) tool. The interactive map will show results from NSW along with the other states for several priority pathogens and different fungicide groups.
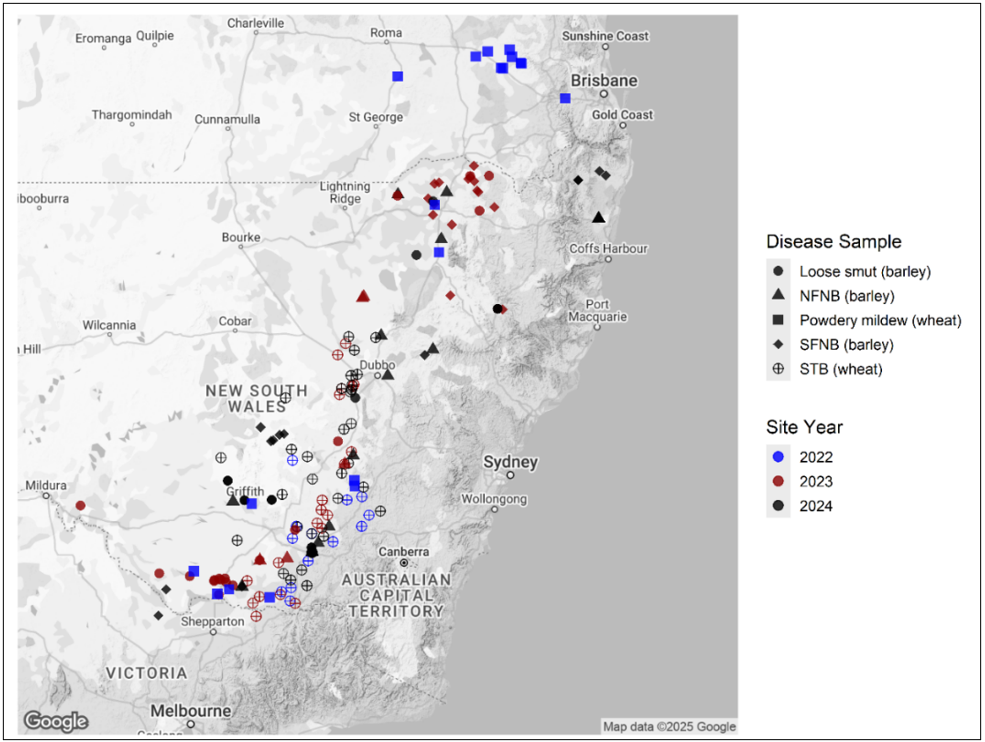
Figure 1. The spatial distribution of samples submitted by NSW DPIRD to CCDM for fungicide insensitivity screening from 2022 to 2024.
Table 1. The status of fungicide resistance of priority pathogens in NSW based on fungicide resistance testing by CCDM, 2022–2024.
Disease | Pathogen | Group 3 (DMI) | Group 7 (SDHI) | Group 11 (QoI) |
|---|---|---|---|---|
Barley loose smut | Ustilago nuda | - | - | - |
Net-form of net-blotch | Pyrenophora teres f. teres | LD, R3 | LD, RS3 | - |
Rusts1 | Puccinia spp. | GH2 | - | - |
Septoria tritici blotch | Zymoseptoria tritici | RS | - | - |
Spot-form net-blotch | Pyrenophora teres f. maculata | LD, RS3 | LD, RS, R3 | - |
Wheat powdery mildew | Blumeria graminis f. sp. tritici | LD, R | - | LD, R |
1 Includes barley leaf rust (Puccinia hordei), barley grass stripe rust (Puccinia striiformis f. sp. Hordei) and wheat leaf rust (Puccinia triticina). 2 Green house detection 3 Further testing and analysis required to further clarify resistance status NOTE: barley powdery mildew carries LD, RS, R to Group 3 (DMI, triazoles) in NSW but will not be discussed in this paper. | ||||
For additional information on fungicide resistance in pathogens not discussed below or from other states, visit https://afren.com.au/.
Mechanisms that cause fungicide resistance within different fungicide groups
Most available fungicide mode of action (MoA) groups registered in Australia are a single site fungicide, meaning they bind to a single target site on an enzyme within the biochemical pathways of a pathogen to effectively control them. Pathogens can mutate, which can change the structure of the enzyme binding site so that the fungicide molecule no longer binds to and deactivates the enzyme as efficiently, or at all. Depending on the MoA group, this can happen in different ways and have varying effects.
Group 3 (DMI, triazoles): Changes occur within the Cyp51 gene (all fungi carry this gene) which limits the effectiveness of the fungicide to bind to and deactivate the Cyp51 enzyme and inhibit sterol biosynthesis. This can occur through mutational changes, over expression of the Cyp51 gene or enhanced (over expression) efflux activity (essentially enhanced transport of the fungicide across the fungal cell membrane and out of the cell). This results in changes to how well the individual fungicide controls the pathogen and is commonly associated with reduced sensitivity. The mutations can affect an individual Group 3 fungicide (DMI, triazoles) active or a group of them more than others. As such, there are differing levels of control achieved across the major DMI active ingredients. Multiple different mutations can occur within a pathogen population.
Group 7 (SDHI): SDHIs inhibit fungal respiration by binding to and deactivating the ubiquinone binding site of the succinate dehydrogenase enzyme. This universal enzyme is important for mitochondrial electron transport and cellular respiration. Single amino acid substitutions in SdhB, SdhC, and SdhD enzyme subunits have been shown to confer resistance to SDHI fungicides and different substitutions can confer varying levels of resistance. Reduced sensitivity to SDHI fungicides has also been linked to the enhanced efflux activity, or transport of the fungicide molecule out of cells.
Group 11 (QoI, strobilurin): QoIs resistance in most cases is conferred by a mutation that results in the substitution of glycine by alanine at position 143 (G143A) in the mitochondrial Cytochrome B (CytB) enzyme complex critical for respiration. This mutation stops the fungicide binding to the enzyme and leads very quickly to field resistance.
The frequency of mutations conferring fungicide resistance will vary across pathogen populations. Populations that carry mutations conferring RS or resistance at a high frequency will likely respond poorly to fungicide treatment under field conditions.
Septoria tritici blotch
Septoria tritici blotch (STB) caused by the pathogen Zymoseptoria tritici, survives on wheat stubble as fungal structures (perithecia) that release airborne ascospores under favourable conditions (greater than 24 hours of leaf wetness), spreading over long distances to infect wheat, durum, and triticale crops. Lesions appear up to 28 days post-infection, producing pycnidia, which are small black structures within tan leaf lesions. Pycnidia generate conidia, a different spore type, which spread via rainfall splash within the wheat canopy, causing secondary infections and amplifying STB outbreaks. Yield losses range between 19-49% (Baxter et al. 2024).
Between 2022 and 2024, 94 wheat samples were sent by NSW DPIRD to CCDM for fungicide insensitivity screening. The 2022-24 results reveal that the G143A mutation, which confers resistance to Group 11 (Qol, strobilurin) fungicides such as azoxystrobin, was not detected in any of the samples submitted from NSW.
However, the G143A mutation has previously been detected in samples from South Australia and Tasmania during 2020 and 2022. These detections should act as a warning for NSW growers to use fungicide resistance management strategies to prolong the effectiveness of Group 11 chemistry against STB.
Unsurprisingly, mutations that confer reduced sensitivity to Group 3 fungicides (DMI, triazoles) were found in most samples sent from NSW. Specifically, the mutation Cyp51 G1 (formerly identified as Cyp51 Isoform 11) was present in Z. tritici from most leaf samples. The mutational frequency range was between 0–100%, with the average frequency in the pathogen population (from 2023 and 2024 data) being 76%, with approximately 50% of the samples tested returning a frequency above 90%. The data indicates a reduction of fungicide efficacy would be occurring in the field. Note: mutational frequencies were not reported for 2022 samples.
This mutation is particularly significant because it leads to elevated levels of reduced sensitivity to some Group 3 fungicides such as tebuconazole, flutriafol and propiconazole. The high population frequency of this is not unexpected, as Cyp51 G1 was the predominant fungicide resistance mutation present in the NSW STB population from a previous study conducted in 2016.
These results support our recommendation that Group 11 (QoI, strobilurin) fungicides remain effective in preventing STB infection in NSW. We continue to advise that if your goal is to specifically target STB curatively with fungicides, it is best to avoid using less robust Group 3 triazole actives like tebuconazole and propiconazole. Instead, opt for alternative Group 3 fungicides such as prothioconazole or epoxiconazole.
Net blotches
There are two forms of net blotch that infect barley: firstly, the net-form of net-blotch (NFNB, Pyrenophora teres f. teres), and secondly, the spot-form of net-blotch (SFNB, Pyrenophora teres f. maculata). Both pathogens are stubble-borne, and additionally NFNB can also be seed-borne.
Issues faced with RS and resistance in net blotch populations to Group 3 (DMI, triazoles) and Group 7 (SDHI) fungicides in Victoria, Western Australia and South Australia are well documented. However, until recently, the status of fungicide resistance in NSW net blotch populations was not clear. Preliminary testing of samples sent to CCDM by NSW DPIRD in 2023 uncovered:
- Group 3 (DMI, triazoles) resistance in NFNB
- Group 7 (SDHI) RS in NFNB
Other data shows:
- Group 3 (DMI, triazoles) RS and resistance in SFNB
- Group 7 (SDHI) RS in SFNB
Preliminary data from 2024 (not all results were available at the time of writing) reconfirmed the 2023 findings and indicate that the geographical spread of the mutations extends across the entire NSW cropping region.
Testing to date has identified the presence of the Cyp51A mutation F489L (which affects DMI fungicides) in 16 of the 17 samples. The data also revealed a mutation in the Sdh subunit C (affecting SDHI activity), identified as SdhC-S135R, which was present in 13 out of the 17 samples.
These results indicate widespread fungicide reduced sensitivity within the NSW cropping region to both Group 3 (DMI) and Group 7 (SDHI) fungicide MoAs. Further testing is required to fully understand the complex between species, mutation location in the subunit and frequency. These findings are not overly surprising, given the extent of resistance issues already recorded in other states.
To avoid crop losses and to prolong the effectiveness of affected fungicide groups, it is essential to select barley varieties with robust resistance to SFNB and NFNB, avoid sowing barley following barley, and choose robust chemistries for disease control while rotating active ingredients.
For the given mutations, it is likely that Group 3 (DMI, triazole) prothioconazole will have greater efficacy than epoxiconazole, which in turn is more effective than propiconazole and tebuconazole. Similarly, for Group 7 (SDHI) fungicides, bixafen is expected to have greater efficacy than fluxapyroxad.
It is very important to avoid the continuous use of Systiva® (fluxapyroxad) as a seed treatment within barley crops to limit the development of further resistance to this active within NSW net blotch populations.
Growers should also be aware that the seed-borne nature of NFNB could also mean that fungicide resistance could be spread through the movement of infected seed if it carries these mutations.
Loose smut
Over the past two seasons, infections of loose smut (Ustilago nuda) in barley have become a developing issue across NSW, driven by increased infection levels and optimal infection conditions at flowering (moist humid conditions and temperatures between 16–22 C). The pathogen survives over summer within the seed, having infected the previous year's crop at flowering, with re-infection occurring after seed germination the following season. Therefore, any loose smut issue within a given year is a function of the disease epidemic experienced in the previous year. Loose smut is characterised by masses of black spores which replace the grain in the infected head.
A total of 32 samples were submitted to CCDM for testing. To date, there is no evidence of reduced sensitivity to the active ingredients tebuconazole (Group 3, DMI, triazoles), carboxin and fluxapyroxad (Group 7, SDHI) and azoxystrobin Group 11 (Qol, strobilurin). At this stage, other active ingredients remain untested. Recent loose smut issues appear to be more related to infection levels in the previous crops from which the seed for sowing was sourced, optimal seasonal conditions for seed infection, lack of seed treatment, or poor seed treatment coverage during application.
To mitigate risks, growers should source seed from crops with visually low loose smut levels and ensure proper application of registered seed treatments with high efficacy against loose smut. Ideally, any seed which has infection levels of >1% should be treated with a full label rate of a quality seed treatment and infection levels of >5% should be replaced with a new seed source. Achieving thorough coverage during seed treatment application is essential to reducing the pathogen load and protecting the next season’s crop from loose smut.
Further information on the relative efficacy of seed treatments against loose smut can be found in WA studies by Andrea Hills and colleagues (Hills et al., 2019).
Wheat powdery mildew
Blumeria graminis f. sp. tritici (Bgt), the causal agent of wheat powdery mildew (WPM), is favoured by prolonged mild and moist weather conditions (15 to 22 °C and relative humidity >70%) and dense crop canopies facilitated by high levels of nitrogen in the soil. These factors facilitate long periods of green leaf retention, moisture, and humidity in the canopy which promote WPM disease development.
A total of 19 WPM samples collected from NSW and southern Qld during 2022 were tested by CCDM. The F136 mutation, also known as a ‘gateway’ mutation, has been previously associated with reduced sensitivity to some Group 3 fungicides (DMI, triazoles). This mutation is normally found together with other mutations that are ultimately responsible for the resistant phenotype observed in the field. Once the frequency of the F136 and other mutations in a WPM pathogen population reach moderate levels, then reduced sensitivity to DMI fungicides is possible under field conditions. Very high frequencies may result in resistance in WPM and fungicide failure under field conditions with some DMI actives. The F136 ‘gateway’ mutation itself does not necessarily result in field failure of fungicide treatments. It is however an initial warning that issues with continued DMI fungicide use exist. The F136 mutational frequency in these WPM samples from 2022 were between 53 and 100%.
The results also confirmed the presence of the Group 11 (Qol, strobilurin) mutation A143 in the samples. This mutation confers resistance to actives such as azoxystrobin. Once the frequency of the mutation exceeds approximately 50% within a field, the population is classed as resistant, and the fungicide will likely not control WPM disease development. In 2022, the A143 mutational frequency in NSW and southern Qld WPM populations was between 7% and 58%.
More recent paddock testing undertaken by FAR Australia and AgGrow in 2024 revealed the presence of the Group 11 (QoI, strobilurin) A143 mutation at an average frequency of 41% (ranging between 0% and 98%). Additionally, the same testing showed the F136 mutation at an average frequency of 100%.
When specifically targeting WPM, Group 11 (Qol, strobilurin) fungicides in regions of high risk should be used judiciously and IDM strategies to promote longevity of this MoA should be applied. There is no known resistance to Group 7 (SDHI) active ingredients, but research has shown that this group of fungicides do not have as high efficacy against WPM (Lopez-Ruiz et al., 2023). CCDM testing of these samples also showed that propiconazole was much less effective at controlling WPM than prothioconazole. Prothioconazole is one of the more effective Group 3 (DMI, triazoles) active ingredients for WPM control.
Further information on WPM fungicide management is available (Trengrove et al., 2023) along with WPM mutation frequency and management work from 2020 (Poole et al., 2022).
Cereal rusts
Research undertaken at Plant Breeding Institute at the University of Sydney (USyd) Cobbitty has uncovered barley leaf rust (BLR, Puccinia hordei) fungicide-insensitive pathotypes which originate from the 5453 P- clonal lineage, first detected in Western Australia in 2001. These pathotypes have since spread across all barley-growing regions in Australia, showing significant insensitivity to a range of fungicide actives in controlled environment studies in a susceptible barley variety. Notably, BLR pathotypes exhibit resistance to eight Group 3 (DMI, triazole) fungicides, even when applied at high field-rate concentrations.
Similarly, the wheat leaf rust (WLR, Puccinia triticina) pathotype 93-3,4,7,10,12, identified in 2020, exhibits insensitivity to recommended high rates of nine fungicide actives.
Despite the resistance to Group 3 (DMI, triazoles), fungicide mixtures like Amistar® Xtra (DMI and QoI), and Radial® (DMI and QoI) offer effective control of both BLR and WLR at recommended field application rates by leveraging combined MoAs. Aviator® Xpro (DMI and SDHI), offered effective control of BLR but not WLR (and is only registered for the control of BLR, not WLR). In these cases, it is the addition of the Group 7 (SDHI) and Group 11 (QoI, strobilurin) which is improving control of rust infections.
More recently, 2 barley grass stripe rust (BGYR, Puccinia striiformis f. sp. hordei) pathotypes (BGYR+ and BGYR+A+) have been reported with insensitivity to DMI fungicides. There are no fungicides registered for BGYR control. For experimental purposes, four DMI fungicides (tebuconazole, prothioconazole, propiconazole, and triadimenol) were tested at recommended high field rates for other diseases and found they were all ineffective against the two BGYR fungicide insensitive pathotypes.
This pathogen complicates management as it infects not only barley grasses but also certain wheat and barley varieties. It must be stressed that the infection on crop cultivars is generally low in severity, and in-crop fungicide management is not recommended.
Cultural practices are key for reducing BGYR risk. This includes controlling barley grass, wheat, and barley regrowth before the cropping season to prevent rust survival, reduce initial inoculum levels, and minimise the risk of rapid disease development.
These WLR, BLR and BGYR pathotypes exist in our natural environment, but importantly, to date, have not been shown to be associated with field failure of fungicide applications. It must also be stressed that there is no known stripe rust (Puccinia striiformis) resistance to any fungicide MoA groups or active ingredients. It is imperative to submit a rust sample to USyd and contact your local pathologist if fungicide field failure is suspected with cereal rusts.
Further information on the research undertaken by USyd on rust fungicide resistance can be found in previous GRDC update paper (Park et al., 2024).
Integrated disease management (IDM)
There are a number of IDM strategies that growers can use to reduce pressure from foliar diseases and minimise fungicide applications including applying the ‘AFREN fungicide 5’ which include:
1. Avoid susceptible crop varieties
Avoid susceptible varieties and select those that provide the best resistance ratings to known or likely leaf disease issues in your region whilst also offering adequate yield potential. This gives cereal crops the best chance of optimising yield in the presence of a pathogen and will reduce the amount of inoculum produced and released within a crop and the broader region (e.g., stripe rust spores). Delaying sowing to the end of a variety’s sowing window in some situations can reduce in-crop infection levels by altering likely environmental conditions at different growth stages, but it is still most important to maximise yield.
2. Rotate crops – use time & distance to reduce disease carry-over
Sow break crops for one or more years between cereal crops. Break crops include pulses, canola and grass free pasture legumes (e.g., lucerne). This will facilitate the breakdown of stubble-borne cereal pathogen inoculum (e.g., STB, yellow leaf spot and net blotches) present within cereal residue retained within a paddock. Grass weed control is vital in break crops as most grass weeds are alternative hosts of winter cereal pathogens. Note that crop rotation is not effective for pathogens such as rust and powdery mildews which have wind-borne spores that can be distributed over long distances.
3. Use non-chemical control methods to reduce disease pressure
- Stubble provides a source of inoculum for necrotrophic foliar diseases such as STB in wheat and SFNB and NFNB in barley. Cutting height at harvest can affect the physical amount of stubble left standing in the paddock for pathogens to further colonise post-harvest. Other reduction management options for stubble-borne diseases include burning, mulching, grazing and baling or soil incorporation of stubble. The risks of moisture loss and erosion must be considered prior to undertaking options such as burning and cultivation.
- Chemical or mechanical control of cereal volunteers and weeds during the summer fallow period is critical to reducing the survival of rusts and mildew pathogens. Controlling the green bridge reduces or breaks the inoculum cycle of these diseases.
- Source seed which is free of or has low levels of pathogens that can be seed-borne (e.g., loose smut and NFNB).
4. Spray only if necessary and apply strategically
Fungicide application can be a complicated process, but growers and their agronomists should consider:
- Has the disease been correctly identified if present in the crop or accurately reported in my local area if wind-borne (e.g., rust)?
- What is the resistance rating of my varieties to these diseases and hence how are they likely to react?
- What is the incidence and severity of infection currently in the crop and what is the growth stage? That is, is it too early (key leaves not emerged) or too late (e.g., APR to stripe rust already active) to spray to achieve return on investment? Is disease incidence and severity very low or severe infection and green leaf area already lost?
- Have weather conditions (rainfall or fog and temperature) in the last 2 weeks and predicted for the next 2 weeks conducive to disease development which can vary between pathogens?
- What are the strengths and weaknesses of different MoA or fungicide actives against the target leaf disease(s) and is there any recorded fungicide resistance issues within the pathogen population in my region that should be considered?
5. Rotate and mix fungicide actives and MoA groups
- Avoid using Group 7 and 11 fungicides in areas where resistance to QoIs and SDHI has been reported, including seed treatments
- Minimise use of the Group 3 fungicides that are known to have compromised resistance
- Rotate Group 3 fungicide actives within and across seasons, do not use the same Group 3 product twice in succession
- Avoid more than three applications of fungicides containing a Group 3 active in a growing season
- Group 11 fungicides should be used as a preventive, rather than for curative control and should be rotated with effective Group 3 products
- Avoid applying Group 7 and Group 11 products more than once per growing season, either alone or in mixtures. This includes in-furrow or seed treatments that have substantial activity on foliar diseases, as well as subsequent foliar sprays. Combined seed and in-furrow treatments count as one application
- Always read and follow product labels.
6. Risk identification prior to sowing
Be proactive instead of reactive. Consult paddock notes, management plans and rotation sequences from previous years to identify known and potential disease issues. Gain an understanding of your underlying inoculum levels through the PreDicta®B DNA based testing method. PreDictaB quantifies a wide range of pathogen levels in your paddock and provides an associated risk level.
7. Grazing
Grazing can be a technique to reduce the incidence and severity of cereal foliar diseases. By grazing the crop, green leaf area is removed along with infected tissue present at the time. Grazing also reduces humidity within the crop by opening up the canopy and allowing airflow, thus creating an environment which is less conducive to development of leaf diseases.
Early crash grazing can be an option to reduce disease pressure. However, be mindful of grazing withholding periods if flutriafol was applied to starter fertiliser at sowing. If taking the grazing crop through to grain harvest, stock must be removed from the crop by GS31 to avoid yield penalties. Note that grazing is not as effective a management strategy if stocking numbers are too low or preferential grazing occurs, leaving infected areas of the crop, which can be less palatable to stock, minimally grazed or ungrazed.
8. Adequate nutrition
Ensure adequatenutrition is applied to optimise crop health and yield potential which is balanced to meet seasonal conditions. Application of too much nitrogen can cause the development of excessive canopy biomass exacerbating some biotrophic foliar diseases (e.g., rusts and powdery mildew). Conversely, low nitrogen levels can exacerbate some necrotrophic foliar disease (e.g., yellow leaf spot and net blotches).
9. In-crop monitoring and correct identification
Inspection of cereal crops for the presence and extent of disease development and the resulting management decisions are vital to economic performance. Missed fungicide spray timings on susceptible varieties can have significant yield penalties in conducive seasons.
Further resistance management advice can be found at https://afren.com.au/.
Importantly, the first step prior to considering the in-crop application of any fungicide is to ensure correct identification. The ability to understand when a fungicide application is not required, is just as important to know when one is required. NSW DPIRD diagnostics testing highlights that around 10-15% of samples and enquiries received annually are incorrectly assumed to be disease when they are related to non-biotic factors (e.g. nutrition, herbicide or frost damage) or simply physiological reactions to stress within cereal plants (e.g. leaf tip necrosis or melanism). NSW DPIRD pathologists can assist with correct diagnosis and management advice to ensure growers only apply fungicides when appropriate.
Sample submission
Tracking pathogen dynamics and fungicide mutational changes depends on the submission of samples for testing. If a fungicide application has subsequent suspicious results regarding the level of efficacy of disease control compared with previous experience, and all application parameters—such as correct product selection, correct rates (for water and fungicide), right plant growth stage, water quality and appropriate weather conditions—have been met, it is strongly encouraged to submit a sample for fungicide resistance testing.
Due to Western Australian biosecurity laws, plant material cannot be sent directly from the east coast to CCDM without the proper permits and packaging. CCDM can provide WA Quarantine-compliant packaging for sample submission, or you can contact your local pathologist for assistance.
For rust samples, prepaid envelopes are available from the University of Sydney, allowing you to send samples at no cost. To request these envelopes, contact the Australian Cereal Rust Survey or consult your local pathologist for guidance. Contact information for CCDM and Australian cereal rust survey can be found under the Useful Links or contact information section of the paper.
Conclusions
Fungicide resistance is a real and pressing issue for NSW cereal growers. Pathogen populations are constantly evolving, making it essential to utilise IDM to extend the effective lifespan of currently registered fungicide MoA and active ingredients. Fungicides play a crucial role in crop protection and will always remain a component of IDM strategies. However, incorporating non-chemical strategies can significantly reduce reliance on fungicide applications.
When developing a fungicide management plan, it is important to rotate between and within fungicide MoA groups, avoid consecutive applications of the same product or active ingredient within a season, and adhere strictly to label requirements, including product rates, growth stage restrictions and withholding periods from grazing or harvest so as not to exceed maximum residue levels.
Before applying fungicides, seek a correct diagnosis from a local pathologist if there is any doubt on the identification. Misdiagnosis is one of the leading causes of unnecessary fungicide applications. Submit samples for testing if fungicide failure is suspected following field application.
Acknowledgements
The research undertaken as part of these projects is made possible by the joint investment of NSW DPIRD and GRDC; investments by GRDC with CCDM, AFREN and FAR Australia. Importantly, growers and their advisers are also critical to the success of this research through access to paddocks, sample submission and engagement. The authors would like to thank them for their continued support.
The authors would like to thank the wider AFREN team for continued efforts in promoting fungicide resistance awareness and best practice management.
The lead author would also like to express gratitude to cereal pathology staff members located at Wagga Wagga and Tamworth for their valued contributions to project work. Additionally, the lead author would like to acknowledge the ongoing support for cereal pathology capacity by NSW DPIRD.
References
AFREN, 2024. Fungicide resistance terminology. Accessed 14/01/2025
Baxter B, Ovenden B, Simpfendorfer S, Milgate A (2024) Septoria tritici blotch – risk and management considerations for 2024. GRDC Update February 2024.
Hills A, Thomas G and Jayasena K (2019). Barley loose smut – control, variety susceptibility and effects on grain yield. GRDC Updates February 2019.
Lopez-Ruiz F, Dodhia K, Chang S, Simpfendorfer S and Trengrove S (2023) Fungicide resistance in wheat powdery mildew. GRDC Updates Wagga February 2023.
Park R, Chhetri M, Ding Y, Baxter B and Dadu H (2024) Cereal rust update. Wagga Wagga GRDC Updates February 2024.
Poole N, Wylie T, Fuhrmann K, Morris B, Dodhia K, Chang S, Lopez-Ruiz F, Simpfendorfer S and The AFREN Team (2022) Fungicide resistance update - national situation and issues for the northern grains region. GRDC Updates February 2022.
Simpfendorfer S, Dodhia K, Chang S and Lopez-Ruiz F (2022) Fungicide resistance in wheat powdery mildew in NSW and northern Victoria in 2020-2021. GRDC Updates February 2022.
Trengrove S, Sheriff S, Bruce J, Lopez-Ruiz F, Dodhia K, Poole N, and Morris B (2023) Fungicide resistant wheat powdery mildew – update on management and resistance testing. GRDC Updates February 2023.
Useful links
- AFREN website
- Australian Cereal Rust Survey
- Barley loose smut – control, variety susceptibility and effects on grain yield
- Cereal rust update 2024
- CCDM website
- FAR Australia
- NSW DPIRD research result booklets
- NSW DPIRD Sowing Guide
- NVT Online
- PRIM fungicide portal
Contact details
Brad Baxter
NSW DPIRD, Pine Gully Rd, Wagga Wagga, NSW 2650
Ph: 0428 294 121
Email: brad.baxter@dpi.nsw.gov.au
X: @BradBaxter1985 or @NSW_AGRONOMY
Steven Simpfendorfer
NSW DPIRD, 4 Marsden Park Rd, Tamworth, NSW 2340
Ph: 0439 581 672
Email: steven.simpfendorfer@dpi.nsw.gov.au
X: @s_simpfendorfer or @NSW_AGRONOMY
Fran Lopez-Ruiz
CCDM, Curtin University, Kent St, Bently, Perth WA 6102
Ph: 08 9266 3061
Email: fran.lopezruiz@curtin.edu.au
Robert Park or Mumta Chhetri
University of Sydney, Faculty of Science
Plant Breeding Institute, Camden, NSW 2006
Ph: 02 9351 8800
Email: Robert.park@sydney.edu.au or Mumta.chhetri@sydney.edu.au
Twitter: @PBiCobbitty
NSW DPIRD podcasts: are now on popular streaming platforms, such as Apple and Spotify. Just search for NSW DPIRD Agronomy. Alternatively, you can subscribe and receive NSW DPIRD podcasts on Soundcloud Stream NSW DPIRD Agronomy | Listen to podcast episodes online for free on SoundCloud.
Date published
February 2025
® Registered Trademark
GRDC Project Code: DPI2207-002RTX, DPI2404-007RTX, FAR2302-001RTX, UOS2207-002RTX, CUR1403-002BLX,
