Diseases in pulse crops during 2013
Author: Jenny Davidson, Rohan Kimber, Larn McMurray, Mick Lines, Jeff Paull, Andrew Ware and Kristy Hobson | Date: 25 Feb 2014
Jenny Davidson, Rohan Kimber, Larn McMurray, Mick Lines, Jeff Paull, Andrew Ware and Kristy Hobson
Take home messages
- Nipper lentils and PBA Rana faba beans require additional fungicide sprays due to ascochyta resistance breakdown.
- Ascochyta blight in chickpea cultivars and breeding line; reactions range from Resistant to MR-MS, requiring different spray strategies.
- Botrytis grey mould and chocolate spot were high risk in 2013 due to heavy canopies and wet winter. Strategic spray programs controlled most of the disease.
- Sclerotinia was widespread in many pulse and canola crops. Sclerotes in grain can lead to rejection at silo. Sclerotes are not toxic to stock.
- Pea Seed borne Mosaic Virus was widespread in faba beans in 2013. Seed to seedling transmission is believed to be low in faba beans but growers should be cautious and source seed with a low level of virus. Research is continuing to confirm the transmission rates in varieties, particularly Nura.
- Lupin producers must be aware of the high risk of lupinosis when grazing lupin stubbles this year.
Ascochyta blight
Lentils
Infection of ascochyta blight on Nipper lentil in commercial crops and in trials was widespread across South Australia and Victoria in 2013, confirming the partial breakdown of resistance in this cultivar, which was first observed in 2010. Lesions on Nipper were more aggressive this season than in previous years, indicating the virulent isolates have crossed with adapted isolates and improved their fitness. Ascochyta blight scores on Nipper were similar to Nugget in most agronomy or breeding trials (Figure 1). In research funded by SAGIT (‘Resistance monitoring of ascochyta blight in lentils’) a number of Ascochyta lentis isolates collected from these trials were tested in controlled conditions and found to be highly aggressive on Nipper. This indicates that a similar disease control program to Nugget should now apply to Nipper i.e. spray at podding ahead of a rain front to prevent pod infection. At two of these trials PBA Jumbo had similar disease levels to Nipper and Nugget, while PBA HeraldXT, PBA Ace, PBA Bolt, PBA HurricaneXT and PBA Blitz maintained resistance at all three sites.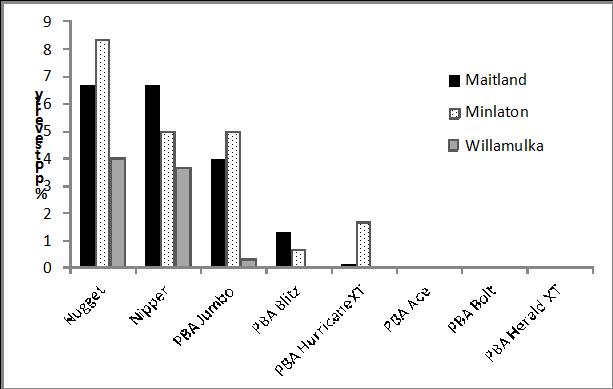
Figure 1. Ascochyta blight scores in lentil NVT trials 2013; lsd = 2.02.
In the SAGIT funded project, lentil stubble infested with ascochyta blight had been collected in December 2012 from five commercial crops (PBA Flash, Nipper and Nugget). This stubble was incubated at SARDI in external conditions, next to pots containing seedlings of eight different lentil lines i.e. resistant sources Northfield, Indianhead and ILL7537; commercial cultivars Nipper, Nugget, PBA Flash and PBA HeraldXT; and a susceptible check, Cumra. For a four week period starting 26 July 2013, lesions that developed on these seedlings were recorded and collected for storage and further research. While the majority of lesions developed on the susceptible lines Cumra and PBA Flash, a small number also developed on each of the resistant sources and on PBA HeraldXT (Figure 2). These results demonstrate that virulent isolates able to overcome all resistant sources already exist in the A. lentis population in South Australia and the resistance in PBA HeraldXT and similar lines is at risk. Further work is continuing in the SAGIT funded project this year to assess all current NVT lentil entries against the Nipper virulent isolates.
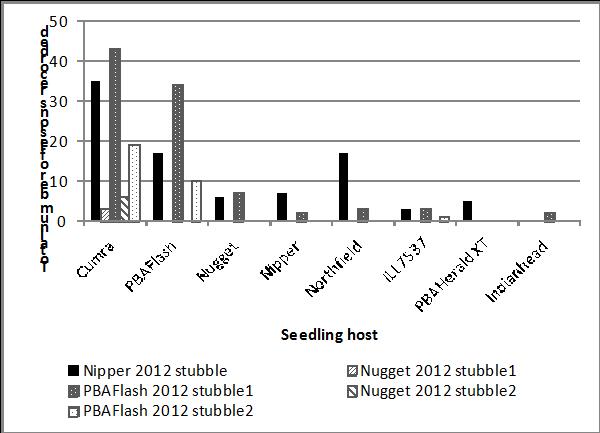
Figure 2. Number of ascochyta lesions recorded on lentil hosts grown adjacent to lentil stubble infested with A. lentis.
Faba beans
Changes in the reaction of ascochyta blight on previously resistant lines of faba bean were observed in faba bean breeding trials in the Lower North region of South Australia in 2013 and in a commercial crop from Poison Gate in NSW in 2012. Isolates of A. fabae from these sites were tested in greenhouse conditions at SARDI. The Poison Gate isolate from NSW was able to infect the previously resistant PBA Rana and a number of breeding lines, indicating virulence against one resistance gene. The isolates from South Australia were able to infect PBA Rana as well as Farah and a range of other breeding lines, confirming that a second resistance gene has been compromised (Table 1). Further research is being conducted to identify resistance to these virulent isolates. Management of ascochyta blight on PBA Rana now involves additional fungicide sprays particularly during podding to prevent pod infection and seed staining. Further research will investigate the benefit of a vegetative spray for ascochyta blight on PBA Rana.
Table 1. Pathogenicity of a standard isolate and Poison Gate isolate of A. fabae in a glasshouse test on 17 known resistant and 4 known susceptible faba bean genotypes in Australian germplasm, and ascochyta scores at two field trials in SA. Potential resistant sources that remain stable in all assessments are marked. Shaded squared indicate susceptible reactions
| Line |
Ascochyta blight severity - stem lesions (%) |
||||
|---|---|---|---|---|---|
| Glasshouse - A. fabae Isolate | Field trial - South Australia | Potential resistant source in pedigree | |||
| Standard | Poison Gate | Saddleworth | Tarlee | ||
| Acc 1322 | 0.00 | 0.00 | - | - | 1322 |
| Acc 622-1 | 0.00 | 0.00 | - | - | 622 |
| AF06125 | 0.00 | 0.67 | 1.67 | 0.00 | 970, S95005/5, 622, 483 |
| AF09169 | 0.00 | 0.83 | 40.00 | 13.33 | IX38/1-10AR, 1269, 483 |
| AF09167 | 0.00 | 1.79 | 26.67 | 13.33 | IX38/1-10AR, 622 |
| AF09059 | 0.00 | 2.13 | 8.33 | 10.00 | 683, 483, 970, S95005/5 |
| AF07125 | 0.00 | 3.47 | 30.00 | 23.33 | 1108, 683, 483 |
| AF08161 | 0.00 | 4.35 | 63.33 | 30.00 | 611, 622 |
| AF05095-1 | 0.00 | 5.52 | 16.67 | 3.33 | 920/3, 483 |
| AF05069-2 | 0.00 | 6.58 | 1.67 | 0.00 | 611, 722, 622, 483 |
| Acc 970 | 0.00 | 10.93 | - | - | 970 |
| PBA Rana | 0.00 | 11.21 | 21.67 | 6.67 | 611 |
| Acc | 0.00 | 24.42 | - | - | 1269, 483 |
| AF09096 | 0.00 | 40.17 | 83.33 | 33.33 | S95005, 483, 611 |
| AF04053 | 0.00 | 41.25 | 81.67 | 35.00 | 683, 483 |
| Nura | 0.75 | 0.60 | 13.33 | 8.33 | 622 |
| Farah | 1.71 | 8.33 | 50.00 | 31.67 | 483 (heterogenic R) |
| Fiesta | 5.75 | 12.50 | 73.33 | 50.00 | 483 (heterogenic S) |
| Doza | 22.52 | 17.29 | 83.33 | - | |
| PBA | 24.37 | 11.16 | 70.00 | - | |
| Icarus-3 | 28.54 | 35.21 | - | - | |
| Legend | R | MR/MS | MS | S | |
Chickpeas
The Southern Region Pulse Agronomy project conducted fungicide trials for ascochyta blight on chickpea at Kingsford in 2013; plots were artificially inoculated with stubble in July. In these trials, there was a strong correlation between the ascochyta blight score and % yield loss, compared to plots with fortnightly sprays of fungicide (Figure 3). GenesisTM Kalkee, PBA Striker and PBA Monarch showed greatest yield loss (≥30%) in plots that were unsprayed or sprayed only at podding. A three spray strategy (strategic treatment) reduced yield loss to 15% in these lines compared to fortnightly sprays. PBA Slasher and PBA Maiden had a similar yield loss (10-15%) in all three treatments. Hence under these conditions a podding spray was sufficient to control the disease, although the current management practice for PBA Maiden is a spray at 6-8 weeks as well as the podding spray. GenesisTM090 showed no yield loss, but the presence of lesions on foliage and stems indicates a podding spray is a good insurance against potential pod and seed infection.
Fifteen chickpea cultivars and breeding lines were scored for reaction to ascochyta blight in a second trial at Kingsford in 2013 (Figure 5). Resistant reactions were noted in CICA1156 and CICA1016, as well as Ambar, Neelam, GenesisTM090, GenesisTM079 and PBA Slasher. As with the first trial there was a good correlation between the ascochyta scores and yield.
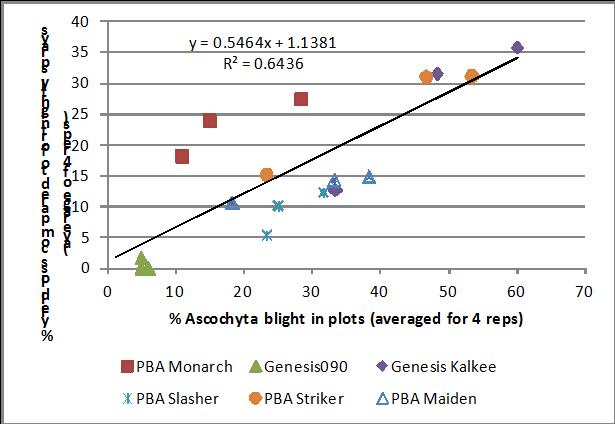
Figure 3. Relationship between % yield loss (compared to fortnightly sprays) and ascochyta blight severity in fungicide trial at Kingsford, 2013.
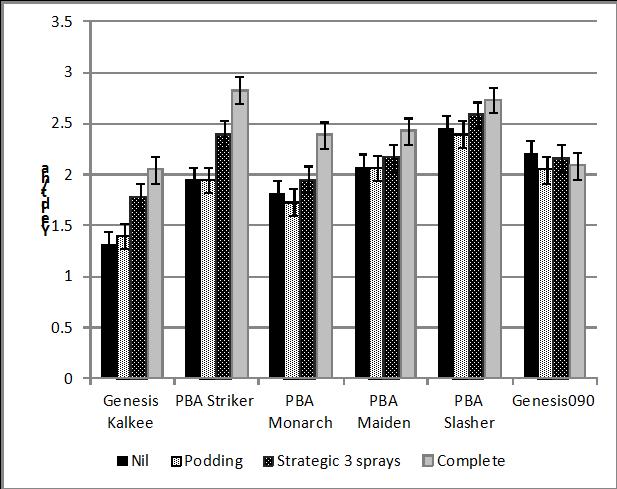
Figure 4. Yield of chickpea lines in ascochyta blight fungicide trial at Kingsford 2013.
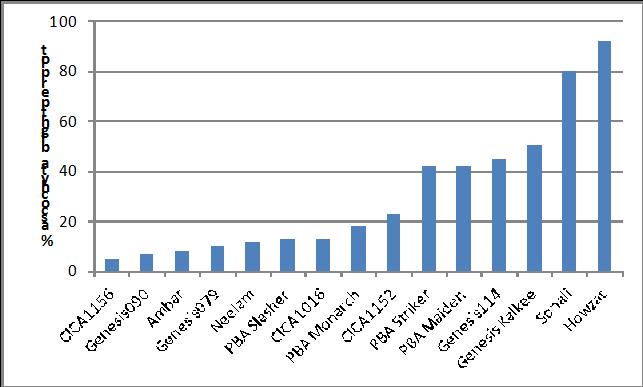
Figure 5. % Ascochyta blight per plot in chickpea lines at Kingsford Sept 24 2013.
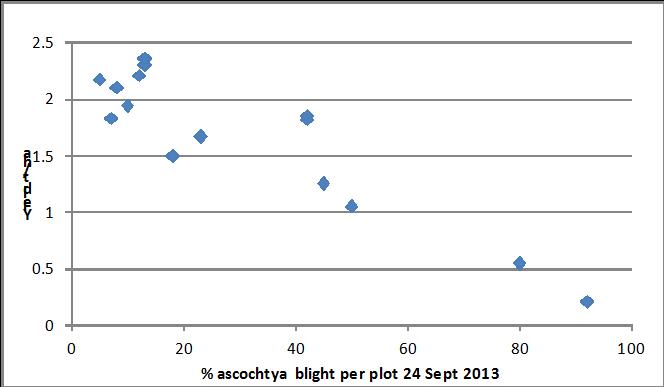
Figure 6. Relationship between ascochyta blight rated on 24 September and final yield in chickpea trial.
Blackspot in field peas
Blackspot Manager forecasted that field pea crops sown during the second and third week of May in 2013 were at risk of blackspot in many areas. This is the peak sowing timing for many field pea crops in South Australia and as a result there were a number of reports of high blackspot infection in pea crops this season. The heavy winter rains further increased this disease to a high level in some crops.
Questions were raised about late (spring) sprays of mancozeb in field peas to control blackspot. This is less likely to be economic if no earlier sprays have been applied. The most economic strategy for blackspot includes a thiram based seed dressing (eg. P-Pickel T®) followed by mancozeb at 9 nodes (canopy closure) and a second mancozeb spray at early flowering. This strategy is likely to only be economic in field pea crops with a potential yield of 2 t/ha or more.
Botrytis and sclerotinia diseases
Prolific early crop canopy development occurred in a number of pulse crops across South Australia due to high winter rainfall and warmer than average May and June temperatures in 2013. Canopy closure sprays for botrytis diseases (chocolate spot and botrytis grey mould [BGM]) were initiated in these crops in late July- early August, which is 3-4 weeks earlier than normal. Consequently multiple sprays were required later in the season to maintain disease control. The dry spring assisted in keeping these diseases under control. PBA Ace had a moderately resistant reaction to BGM in trials in the last season while PBA Bolt and PBA HurricaneXT are moderately susceptible and required additional fungicide sprays after the canopy closure spray, particularly when conditions are moist or humid.
The wet winter also encouraged sclerotinia in many pulse crops and in some canola and lupin crops during August and September, with BGM and sclerotinia occurring together in many cases. BGM severity was increased where wild tares acted as disease foci, and sclerotinia appeared worse where canola had been grown recently in the rotation. Sclerotes were observed in some grain samples, particularly lupins and canola. If contaminated grain is used as seed for cropping, the sclerotes will transfer the disease into the new crop. Grain contaminated with sclerotes is safe to use for stock feed.
Extensive rainfall also encouraged white leaf spot in some canola crops during August and September. The symptoms are similar to blackleg lesions on the leaves, but without the pycnidial black spotting in the centre. White leaf spot rarely results in yield loss.
Downy mildew trials in field peas
Treatments for downy mildew in field pea were tested in a trial at Kybunga in the mid north of SA. This included ApronXL®, and three other seed treatments, and a foliar treatment of phosphorous acid (Agri-Fos 600), at 3.5 L/ha, applied at three different timings. No differences in yield were observed between treated and untreated plots suggesting the level of disease (leaf infection up to 25% per plant on August 8th), was insufficient to cause a yield loss last season. No seedling death or stunting was noted in this trial. Downy mildew is most damaging when young seedlings are emerging, resulting in death of seedlings and stunted growth. A metalaxyl seed dressing is recommended in at risk paddocks to prevent this early damage.
PSbMV in faba beans
Staining from Pea Seedborne Mosaic Virus was widely detected in faba bean grain harvested across South Australia and Victoria this season. Early results from breeding trials suggest that Nura had the highest rate of infection compared to other varieties. This virus is transmitted between plants and crops by aphids, most commonly by green peach aphid (Myzus persicae), but also by the oat/wheat aphid (Rhopalosiphum padi). The latter was abundant in cereal crops this year and may have been responsible for virus transfer.
The source of the virus may be any pulse crop or pulse pasture but not cereals nor canola. Often the source of PSbMV is from neighbouring field pea crops, especially Kaspa which is highly susceptible. It is unclear why the PSbMV level appears to be low in field pea this season.
The transmission of this virus from seed to seedling is thought to be low in faba beans (<1%), which means the grain can be used to sow the next year’s crop with low risk of infection. Further studies will be conducted to confirm the low rate of transmission in different faba bean varieties, especially Nura. However note that if using infected field peas, the transmission may be 100% i.e. every infected seed may produce an infected seedling.
Growers who are concerned about virus transfer can reduce the risk by;
- sourcing seed from unaffected area or region or use seed from a less affected area of the crop,
- grading hard to remove smaller grain, which are more likely to be infected,
- using imidacloprid (Gaucho®) seed dressing to prevent aphids colonizing the seedling crop in autumn – early winter, and spreading more virus,
- planting into a stubble to reduce aphid landings during autumn- early winter,
- avoiding planting faba beans adjacent to field peas especially KaspaA which may be a reservoir for PSbMV; and
- if aphids colonise crops, spray in spring before the aphids take flight.
Other staining issues have also been recorded on faba bean grain, leading to downgrading or rejection at the silo. These are a mixture of ascochyta blight (Ascochyta fabae), chocolate spot (Botrytis fabae), weather staining and possibly frost damage. Grain with more than 5% infection of Ascochyta fabae and/or more than 10% infection of Botrytis fabae is not recommended for sowing.
Lupinosis risk in lupin stubble
Lupin producers must be aware of the high risk of grazing lupin stubbles this year. Lupinosis is caused by toxins produced by the fungus Phomopsis leptostromiformis (synonym Diaporthe toxica). This fungus grows saprophytically in mature lupin stems. Varietal resistance will slow the growth of the fungus, but won’t stop it, so under moist and humid conditions even the most resistant variety currently available can produce toxins with the potential to kill stock. Conditions towards the end of the 2013 growing season were ideal for the development of the Phomopsis fungus. Lupin growers wanting to graze stubbles are advised to inspect the stubble for symptoms. These usually appear on senescing or dry lupin stems as dark purplish brown lesions which bleach with age and contain black fruiting bodies. Lesions can develop on pods, causing the surface of green pods to become 'slimy' and mature pods to be shriveled with dark discolouration.
Contact details
Jenny DavidsonGPO Box 387 Adelaide, 5001.
08 8303 9389
Was this page helpful?
YOUR FEEDBACK
