Developing a field assay kit to detect glyphosate resistance
Author: Dale Shaner, USDA-ARS (retired), USA | Date: 26 Feb 2013
Dale Shaner, USDA-ARS (retired), USA
Take home message
A field kit to detect glyphosate resistance in the field is being evaluated by Monsanto.
The kit will be used as an initial screen for the presence of glyphosate resistant weeds.
Introduction
Herbicide resistance is a major concern in crop production. There is widespread resistance to Group A and Group B herbicides in a number of species. However, the selection of glyphosate resistant (Group M) weeds has increased the need to manage herbicide resistant populations. One of the first steps in managing herbicide resistance is identifying resistant populations early. There are multiple methods for identifying herbicide resistant weed populations, but many of the methods are time consuming and laborious. If we could develop a field kit that could reliably detect glyphosate resistance in the field within a short time period (1-2 days) then a grower might be able to destroy the population before it became widespread. Monsanto scientists have developed a colorimetric method that has potential for such a kit, although it is not presently available. In this short paper I will describe the principal behind this kit and the progress that has been made to date.
Principles for detecting glyphosate resistance
Glyphosate kills plants by inhibiting an enzyme in the biosynthetic pathway that produces the essential amino acids phenylalanine, tyrosine and tryptophan. This enzyme is called 5-enolpyruvylshikimate-3-phosphate synthase or EPSPS, for short (Figure 1). The inhibition of EPSPS stops the flow of carbon through this pathway and results in the rapid accumulation of shikimate. Shikimate builds up to extremely high levels in the plant and can be detected (Figure 2). However, the current method of detection requires the use of a spectrophotometer that reads in the ultraviolet. The colour cannot be detected by eye.
Monsanto has developed an alternative method to detect shikimate that can be detected by eye. This method utilizes a coupled reaction between an enzyme called shikimate dehyrdogenase (SHKD) and diaphorase. This coupled reaction reduces a dye from a colourless to a coloured material and the amount of colour is proportional to the concentration of shikimate in a plant extract (Figure 2).
This method to detect glyphosate resistance is based on differences in the accumulation of shikimate in susceptible versus resistant biotypes. Susceptible biotypes will accumulate shikimate at low glyphosate concentrations, whereas resistant biotypes will only accumulate shikimate at very high doses (Figure 3). This difference appears to be true for most glyphosate resistant weeds.
Glyphosate resistance assay
The assay works by taking leaf discs from the young, rapidly expanding leaves of the weed. Leaf discs are incubated in different concentrations of glyphosate for 24-48 h under light and then shikimate is extracted from the leaf tissue by freeze-thawing. The shikimate level in the extract is measured using the SHKD assay. The amount of shikimate accumulated at a low concentration of glyphosate is then compared to levels accumulated at a high concentration of glyphosate (Figure 3).
Strengths and weakness of the assay
This assay has been field tested by researchers within the U.S. and the preliminary results look promising. However, there is still a lot of research that has to be done before the assay is available for widespread use.
The assay, as it currently exists, can only be done by an experienced researcher. The biggest weakness of the assay is picking the right plant material to assay. Shikimate accumulates in leaf tissue that is young and vigorously growing. This means the assay can only be done on relatively young plants in the field. For Lolium the rapidly growing tissue is at the base of the plant where the growing points are located or in the newest emerging leaf coming out of the whorl. If the plants are tillering, then a young tiller will also work. Taking tissue from a mature leaf or an old plant will not work because there is limited shikimate accumulation occurring in mature tissue.
The assay needs to be conducted under light during the incubation. The assay also requires access to a freezer or dry ice to do the freeze-thawing. In addition, the assay will have to be optimised for each new species tested. Initially a tester would conduct the assay on known susceptible and resistant biotypes to become familiar with the assay and to determine if it is going to work.
The assay uses enzymes as well as NADP, which are both relatively unstable. Initial work has shown that the enzymes and NADP can be dried and then reconstituted in the field, but the dried enzymes/cofactors have limited shelf life.
The strength of this assay is that it is colorimetric and, once the kit has been optimised, should provide another tool for rapidly detecting glyphosate resistance.
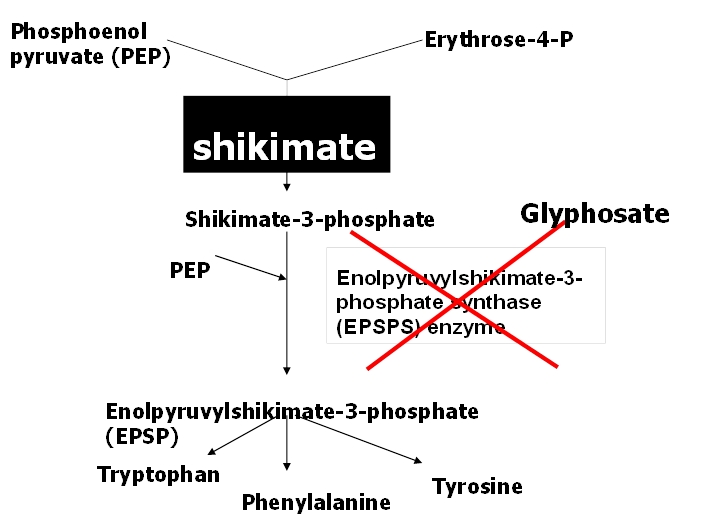
Figure 1: Aromatic amino acid pathway and site of glyphosate inhibition
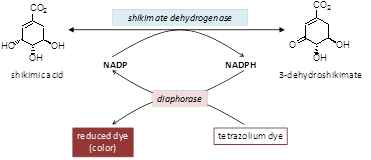
Figure 2: Couple reaction between shikimate dehyrogenase and diaphorase
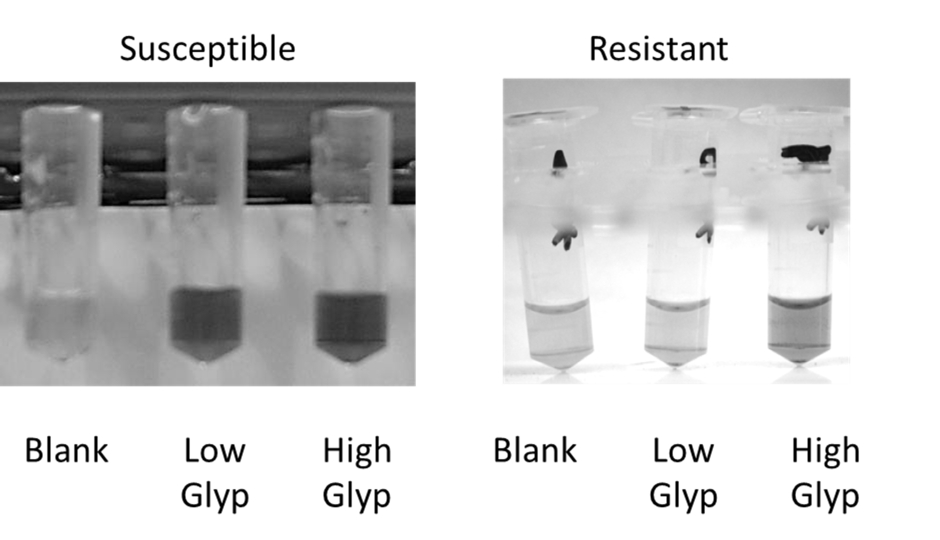
Figure 3: Detection of glyphosate resistance by colorimetric assay
Contact details
Dale L. Shaner
USDA-ARS (retired)
Phone: 01-970-556-4826
Email: daleshaner@aol.com
Was this page helpful?
YOUR FEEDBACK
