Climate controlled the Russian Wheat Aphid invasion in 2016
Take home messages
- Russian wheat aphid (RWA) is a significant new pest of Australian cereal crops and has been found in SA, Vic and NSW in 2016.
- In the winter and spring of 2016 the rain and subsequent entomopathogenic fungus development controlled the aphid efficiently.
- RWA should be a manageable pest, with the use of a combination of effective cultural, chemical and biological controls available.
- Plant resistance will be the most efficient solution, but breeders need to develop varieties adapted to Australian growing conditions
Occurrence and current distribution in Australia
Russian wheat aphid (Diuraphis noxia, RWA) is one the world's most economically important pests of wheat, barley and other cereal grains. It is native to southern Russia, the Middle East and Central Asia, but has spread to other major grain producing regions in Europe, Africa, North America and South America. RWA is primarily associated with cereal production regions characterised by warmer, drier climates.RWA was first reported on the 13 May 2016, identified by SARDI entomologists in a wheat paddock near Tarlee in the SA Mid North region. Further surveillance has led to the detection of the species across the eastern half of South Australia, western and central Victoria, and southern parts of NSW. The large geographical spread of RWA suggests that the aphid had become established in Australia and gone unnoticed for several years.
Identification
Wingless adults grow up to about 2mm long and have distinctively short antennae. They are light lime-green in color, with only the tips of the legs and antennae that are black. While the bodies of established cereal aphids (Oat and Corn Aphid) are often pear or globe-shaped, RWA is longer and more spindle-shaped and lacks cornicles (commonly referred to as ‘exhaust pipes’). Adult RWA have two cauda or a double ‘tail’ at the rear end of the aphid. These structures are difficult to see on RWA with the naked eye and can be seen best when viewing the aphid from a profile perspective under a microscope (Figure 1 and 2).
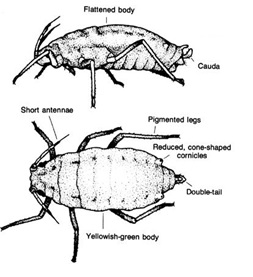
Figure 1. Diuraphis noxia morphology (Source: Igienica Sassarese).
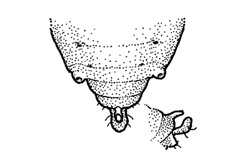
Figure 2. Russian Wheat Aphid abdomen with double ‘tail’ and very short cornicles (Source: University of Nebraska).
Adult RWA can be winged (alates) which are also up to 2mm, with longer antennae and the body is generally darker in colour.
Cycle
In its native range, the annual lifecycle of RWA includes sexual and asexual phases. However, like most other introduced aphid pests in Australia, invasive populations of RWA have so far only been observed reproducing asexually with females (‘virginoparae’) giving birth to live female offspring.
Host range and plant damage
The host range of RWA includes more than 140 species of cultivated and wild plants within the family Graminae (grasses). These include wheat, barley, triticale, rye, oats, pasture grasses and wild genera including Poa, Bromus, Hordeum, Lolium, Phalaris and others. Wheat and barley are the most susceptible cultivated plants, while triticale, rye and oats are less susceptible. This wide availability of host plants partly explains why RWA has a history of successfully invading new regions.
Unlike other cereal aphids that damage plants by removing nutrients, RWA also injects salivary toxins during feeding that cause rapid, systemic phytotoxic effects on plants, resulting in acute plant symptoms and potentially substantial yield losses. Even a few aphids can cause plant damage symptoms to appear as early as seven days after infestation. These include:- White and purple longitudinal streaks on leaves.
- Curled, rolled or hollow tube leaves.
- Stunted growth or flattened appearance.
- Discolored leaves.
- Hooked-shaped head growth from awns trapped in curling flag leaf.
- Bleached heads.
The salivary toxins injected by RWA during feeding damages plant chloroplasts, resulting in reduced photosynthetic ability, delayed leaf initiation and tillering and reduced numbers of fertile tillers, shoot and root biomass, grains per ear and grain weight. Yield impacts are determined by the percentage of infested tillers and plants and crop development stage. Heavy infestations during early growth can cause serious damage (under USA conditions). From early booting to the soft dough stage, feeding on upper leaves, in the leaf sheath and next to the developing head, can cause direct yield losses. In wheat and barley, damaged leaf tissue does not recover. If aphids are controlled, new growth proceeds normally (new root and shoots are unaffected) and plants may recover unless excessively stressed. After soft dough stage, further impact is thought to be minimal.
Population dynamics
In autumn, aphids may infest wheat or barley seedlings soon after emergence, either from wingless aphids walking off nearby senescing hosts or from migrating winged aphids. Early in the crop cycle, the vast majority of aphids are wingless. In cereal regions of the world where RWA causes problems, populations start to increase from tillering and stem elongation. Aphids can move by walking among leaves, tillers and plants, so that the percentage of infested plants increases during the crop cycle. Population growth often becomes most rapid from booting onwards. Aphids feed in dense colonies, typically at the base and sheath of younger leaves and within leaves curled by their feeding. Aphids prefer the newest leaves of plants, and are often found on the last two leaves unfurled.
Later in the crop cycle as aphid population density increases, the proportion of winged aphids increases and may reach high levels prior to ripening; at this stage, aphids emigrate in search of alternative summer hosts. Alate RWA are weak flyers, but are thought to travel on wind currents efficiently enough for some aphids to locate isolated host plants.
RWA are able to survive under a wide range of temperatures, the optimum temperature range is considered to be around 18 to 21°C. RWA does not do well under higher temperatures ( greater than 25°C). Generation time ranges from approx. 20 days at 10°C, and nine days at 20°C.
Like other aphids, populations of RWA are strongly regulated by environmental conditions. Survival of aphids is affected by exposure to rainfall, drying winds, predators and parasitoids. Rainfall washes aphids from upper leaves and heavy rainfall may cause mortality of up to half of the population.
2016 RWA dynamics in Australia
The obvious question is why this sudden outbreak of RWA occurred in 2016. RWA infestations at the ‘initial’ outbreak in SA appeared to have commenced on wheat volunteers. The early onset of rain in January 2016 may have created a ‘green bridge’ as many of the heavier ‘paddock wide’ infestations were in early sown crops that had volunteer cereals and many of the earlier infestations in other parts of SA and Victoria were first detected on volunteers. In other later-sown and/or less infested paddocks, the RWA attacks were mostly limited to the field borders, where local migration from weedy grasses into the paddock occurred.
In August 2016 SARDI set up a GRDC-funded research program to monitor population dynamics in the field. Four unsprayed paddocks were observed in each of four cereal districts in RWA affected regions of SA and Victoria (PIRSA 2016). This project showed that the aphid populations were very different among regions (from 0 to up to 160 aphids/100 tillers), but declined everywhere during the month of September. Rain washed off the aphids and those surviving, hidden in the rolled leaves, were then attacked by entomopathogenic fungi and died (Figure 3).
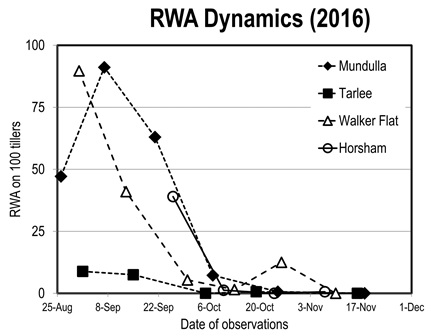
Figure 3. Russian Wheat Aphid population dynamics in an unsprayed paddock in four regions of SA and Victoria (average of four paddocks in each region).
This sharp population decline was clearly unexpected, since in most areas worldwide RWA does build up in spring and does most damage when attacking flag leaves and causing head abortion. The unusual weather of 2016 might have been both the cause for the RWA rise and the solution.
Yield losses in 2016
In this same trial some areas were sprayed. Symptoms continued to develop for one or two weeks after aphids were sprayed, but then plants grew ‘through’ and seemed to recover and develop normally. Some white tillers were observed but they were low in number. Harvest estimates showed no significant differences between unsprayed and sprayed areas in any of these paddocks, except for a very heavily infested durum wheat paddock (with greater than 50% of tillers showing symptoms in late September).
Insecticides
Since RWA had not previously been present, no pesticides were registered against this pest in Australia. In June 2016 SARDI initiated insecticide trials in order to obtain emergency permits. Following trials chlorpyrifos and pirimicarb are now listed for control of RWA under Emergency Use Permit APVMA 82792. Other (non-emergency) permits will follow, but there are good reasons to hold off spraying until thresholds are reached because:- Insecticides will reduce numbers of predators and other beneficials, which is likely to result in a spike in numbers of RWA (and other aphids) later in the year.
- Foraging honeybees will succumb to sprays and must be protected. Speak to local beekeepers.
- Spraying can foster resistance in pests, so must be used only when required.
Preliminary trials conducted by cesar suggest that seed treatments currently registered in Australia to control cereal aphids are likely to be effective against early RWA colonisation. North of the initial detection site of RWA, using an Agri Link seed dressing trial site, SARDI researchers assessed aphid abundance and tillers for symptoms on 31 May 2016, in plots with and without Gaucho seed dressings. Seed treatment gave effective control of aphids moving into these plots from surrounding buffers where aphids were present and symptoms observed. This is supported by field reports from advisers and growers in 2016 in numerous parts of southern Australia. Products containing imidacloprid, thiamethoxam and clothianidin are likely to prove effective, although thorough trials are needed to fully understand role of seed treatments in managing RWA. Based on overseas experience, it is unlikely that fipronil-based products will effectively control RWA (Wilde et al. 2001). At this stage, the length of protection offered by registered cereal seed treatments remains unknown.
Management in 2017
The very wet spring, complemented with some high early-summer rainfall events (December 2016), suggest that in this coming season there will again be a serious risk of RWA populations building up on volunteer cereals and other grass hosts. Controlling aphid populations and avoiding such a green bridge effect in late summer will help reduce RWA populations. Growers should pay particular attention to cereal volunteers, pasture grasses and wild genera including Poa, Bromus, Hordeum, Lolium and Phalaris. If possible aim to keep paddocks ‘Free for February’ and avoid direct drilling combined with herbicide spraying of volunteers. While a longer break will give improved control as well as provide other benefits (such as water and nutrient retention), if paddocks were kept bare for February at a minimum, the risk of green bridge issues involving aphids would be substantially reduced.
Other practices such as later planting of winter cereals to delay and reduce early aphid infestation and agronomic practices to promote crop vigour and dense canopy growth, will inhibit RWA populations and reduce their impact on cereals.
Post-emergence monitoring
Aphids may infest crops during any stage of crop development, from early establishment to maturating flag leaf. Check crops regularly following seedling emergence. RWA are often difficult to find when at low numbers so check for the characteristic and distinctive leaf streaking and rolling. Infestations often begin along crop edges, usually on the windward side or adjacent to infested grasses. RWA also commonly occurs in areas of paddocks where plants are sparse, on sandy rises or adjacent to bare ground. After initial infestation, aphids can rapidly spread across a paddock.
Chemical control of RWA is effective; however decisions on the need for foliar treatments should be based on the proportion of seedlings or tillers infested. Economic thresholds (ET) from international literature provide a useful guide but these will be situation dependent and trial work is required to validate locally. Yield loss under Australian conditions, crop yield potential and cost of the chosen control option are all determining factors. Threshold guidelines recommended in the USA vary somewhat between regions, but for early season growth current recommendation is an ET of 20% seedlings infested up to the start of tillering and 10% of tillers infested thereafter. Local research will be required to test, and if required, to modify these thresholds for Australian crop conditions.
Role of beneficials
RWA is attacked by a range of natural enemies in other parts of the world, many of which also attack other aphids. Of these, groups that commonly occur in Australia include the minute parasitoid wasps Aphidius colemani, A. ervi, Diaeretiella rapae and generalist predators including ladybird beetles (for example, Coccinella spp., Hippodamia spp.), lacewings (Chrysopa spp.), damsel bug (Nabis sp.), hoverflies (Syrphus spp.) and also entomopathogenic fungi. Mummified RWA by the attacks of mainly D. rapae and A. colemani (Heddle et al., 2016) have already been observed. Entomopathogenic fungal diseases, stimulated by high rainfall, seem to have had a substantial contribution to the unexpected sharp decline of populations in spring 2016.
The use of prophylactic sprays for managing invading or dispersing RWA is not advocated. These sprays can create secondary pest outbreaks (such as other cereal aphids) by removing beneficial species. If spraying is warranted, aim to use the softer chemistry to maintain predators and beneficial populations.
Plant resistance
Plant resistance has been deployed as a major management strategy in all areas of the world where RWA is a serious risk. Plant resistance is available, but unfortunately the aphid has also developed new biotypes attacking these resistant plants. A GRDC funded project is underway at SARDI to determine what biotype of aphid has entered Australia, and once this information is acquired, breeders will have information on effective resistance genes. Some initial work has been done in Australia and there are early indications that there may be variability for resistance to RWA in commercial varieties and germplasm carrying resistance genes is available, but plant breeding would take some years to obtain new adapted varieties.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC — the author would like to thank them for their continued support. The work presented here was funded through the GRDC project (DAS00170, Service Agreement 9174815).
The authors would also like to thank Javid Muhammad and Piotr Trebicki (Agriculture Victoria, Horsham) for the Victorian observations, Helen deGraaf, Thomas Heddle, Farah Al-Jawahiri, Ken Henry, Bill Kimber and Michael Nash for the SA observations and all growers for their support.
We also acknowledge Garry McDonald, Julia Severi and Kym Perry for their valuable contributions to an earlier version of this paper and the Plant Health Australia RWA national technical group.
Useful resources
Crop Aphids: The back pocket guide. GRDC
GRDC Paddock Practices - Monitor RWA numbers closely over winter
PIRSA (2016). Cereal Aphid dynamics
PIRSA Russian wheat aphid information
PestFacts newsletter Russian wheat aphid
Plant Health Australia – Threat Specific Contingency Plan
References
Damsteegt, V.D., F.E. Gildow, A.D. Hewings, T.W. Carroll (1992) A clone of the Russian wheat aphid (Diuraphis noxia) as a vector of barley yellow dwarf barley stripe mosaic, and brome mosaic viruses. Plant Dis., 76 (1992), pp. 1155–1160
Heddle, T., M. van Helden, M. Nash (2016). Parasitoids of the Russian Wheat Aphid in Australia. Poster presented at the Australian Entomological Society 47th AGM and Scientific Conference and Entomological Society of New Zealand – 2016 Conference, Melbourne Nov 27-30 2016.
Hughes RD. (1996) A synopsis of information on the Russian wheat aphid, Diuraphis noxia (Mordwilko).(Revised edition). CSIRO Australia Division of Entomology Technical Paper. 1996(34).
Pike KS, Allison D. Russian wheat aphid. Biology, damage and management. Pacific Northwest Cooperative Extension Publication. 1991(PNW371).
Wilde GE, et al. Seed treatment for control of wheat insects and its effect on yield. Journal of Agricultural and Urban Entomology 18: 1-11. 2001
Contact details
Greg Baker or Maarten van Helden
SARDI
Waite Building, Waite Rd., Urrbrae, SA.
08 8303 9536
bill.kimber@sa.gov.au
@PestFactsSARDI
Paul Umina or Lisa Kirkland
cesar
293 Royal Parade, Parkville, VICTORIA
03 9349 4723
pestfacts@cesaraustralia.com
@PestFactscesar
GRDC Project Code: DAS00170, Service Agreement 9174815,
Was this page helpful?
YOUR FEEDBACK
