Investigations into the soil microbiology associated with subsoil manuring
Take home messages
- Bacterial and fungal communities change within two weeks after subsoil manuring, and they remain different from populations in unamended soil for at least two years.
- Soil physical, chemical and biological properties of the ameliorated subsoil become similar to the more fertile, better-structured topsoil.
- Stimulation of the native community by the amendment feedstock appears to be more important than introduction of novel microbial inocula.
- Further investigation will reveal key microbial species and their functional roles after subsoil manuring to establish how they link to soil physicochemical characteristics and crop performance.
Introduction
In the high rainfall zone of southern Australia, dryland crop yields are often constrained by naturally dense clay subsoils that are poorly structured, restrict the movement of air and water and impede root growth (Zhang et al. 2006; MacEwan et al. 2010). A decade of research has shown that subsoil manuring — the incorporation of large volumes of nutrient-rich organic matter into the subsoil by deep ripping — can significantly improve these hostile subsoils and increase crop yields due to sustained changes in the soil physical, chemical and biological properties (Clark et al., 2007; Gill et al., 2008; Sale et al., 2013).
The soil microbial community is hypothesised to play a key role in the amelioration processes that occur after subsoil manuring. This is because microorganisms are recognised to have essential roles in the functioning of soil, including decomposition and mineralisation, formation and stabilisation of structure, nutrient cycling, plant interactions and organic matter formation (Lehman et al. 2015; Murphy 2015). This project aims to uncover the main microbiological processes involved in subsoil manuring and establish a relationship between the soil microbial communities, soil physicochemical characteristics and crop performance. Increasing our mechanistic understanding of subsoil manuring will enable us to address some of the unanswered questions regarding subsoil manuring.
Namely — which amendments work and why? Are organic amendments superior to inorganic amendments? And, do the microbes introduced via the organic amendment enhance the amelioration process?
Method
In order to investigate the soil microbiology associated with subsoil manuring, a series of field sampling and controlled environment experiments are being conducted. The field sampling experiments involve the analysis of a range of sites that have been treated with different amendments at different times and using different methods of incorporation. Controlled environment experiments will enable more detailed investigation and manipulation of the soil type, amendment, crop and duration of incubation.
In each experiment, analysis of the soil microbiological community is being undertaken to identify changes in structural and functional diversity and abundance over time. These microbial data are being paired with soil physicochemical measurements and measures of crop performance. Samples are taken across space and time to capture changes in these after manuring, relative to where the amendment was placed. This methodology enables us to examine the relationships between plant-soil-microbial-amendment factors after subsoil manuring and identify the key drivers of improved yields.
Results and discussion
Change in soil microbial communities
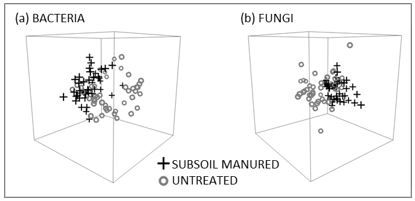
Subsoil manuring changes the diversity and abundance of the bacterial and fungal communities in the subsoil. The microbial community, after subsoil manuring with 20t/ha poultry litter, was found to be significantly different from that of the untreated subsoil (Figure 1).
Figure 1. Bacterial (a) and fungal (b) communities two years after a sodic clay subsoil was amended with 20t/ha poultry litter. Each data point in the ordination represents a microbial community from a subsoil manured or untreated soil sample. Distance between points denotes ecological distance between samples. The two treatments separate into clusters that are significantly different from each other (P<0.001).
Significant differences in soil microbial communities between treated and untreated subsoil have been detected within two weeks of the incorporation of the amendment (data not shown) and they remain divergent for some time after treatment. In this case (Figure 1), soil samples were taken in spring 2015 nearly two years after the incorporation of the organic amendment in summer 2014. This corresponds to the duration of the response seen in the field. Significant differences in soil structure and crop yields have been recorded 3-5 years after treatment (Gill et al. 2012; Sale et al. 2013), indicating that subsoil manuring is able to semi-permanently alter soil conditions and plant growth.
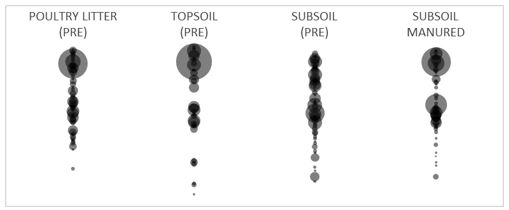
Analysis of the diversity and abundance of the microbial communities indicates that subsoil manuring alters the bacterial and fungal populations so that they become more similar to those found in the topsoil (Figure 2).
Figure 2. Bacterial community fingerprints from the poultry litter, topsoil and subsoil prior to incubation and from the subsoil-manured treatment (subsoil + 20t/ha poultry litter) after incubation for 146 days. Different bubbles represent unique species and the size of the bubble is proportional to its abundance in the community.
Further analysis is required to identify the key species that are changing and their functional roles, but this finding supports observations from earlier experiments where we see changes in soil properties of the hostile subsoil to more closely resembling the topsoil, for example, higher fertility, more porous structure and higher biological activity (Clark et al. 2007). Given the physical and chemical changes that are observed in the subsoil and the measured effect of subsoil manuring on the microbial community, it is suggested that soil microbiology is involved in processes such as the decomposition of the amendment, formation and stabilisation of aggregates, nutrient cycling and plant growth promotion.
Effect of amendment type
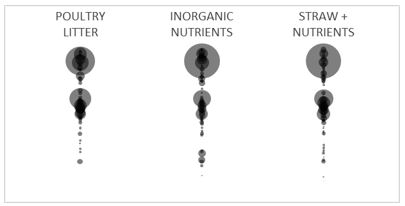
The quality or chemical composition of the amendment used for subsoil manuring may be more important than simply choosing an organic amendment over an equivalent inorganic source. Different amendments with equivalent total nutrient content resulted in similar shifts in the microbial community structure in a sodic clay subsoil, regardless of whether the amendments were organic (poultry litter), inorganic (fertiliser blend) or a mixture of the two (wheat straw plus fertiliser blend) (Figure 3).
Figure 3. Bacterial community fingerprints from subsoil incubated for 146 days with 20t/ha poultry litter, equivalent inorganic nutrients and wheat straw plus equivalent inorganic nutrients. Different bubbles represent unique species and the size of the bubble is proportional to its abundance in the community.
Note that the inorganic nutrient (fertiliser blend) treatment did not contain any carbon, whereas the poultry litter and wheat straw plus nutrient treatments had equivalent total carbon. These results suggest that the chemical composition of the amendment — the rate of nutrients supplied — is a key driver of microbial community structure after subsoil manuring. The apparent lack of effect of carbon source and rate on microbial community structure is puzzling and warrants further investigation with higher resolution microbiological techniques.
Comparison of the three amended subsoils (Figure 3) to the raw poultry litter pre-incubation (Figure 2) reveals that, in this case, the poultry litter did not contribute any unique microbial species to the poultry litter-amended subsoil. All the same species occur whether the soil was amended with poultry litter, inorganic nutrients or wheat straw and nutrients. In other words, stimulation of the native community by the amendment feedstock appears to be more important than introduction of some novel microbial inoculum. This requires further study but potentially opens up more options for alternative amendments to poultry litter that may offer the same resources to the plant and to the soil microbes, but at much lower cost than poultry litter.
Conclusion
Results to date show that subsoil manuring rapidly and significantly affects bacterial and fungal communities in the soil, and that these changes remain for some time after treatment. Amelioration of the subsoil semi-permanently alters the physical, chemical and biological properties to more closely resemble the topsoil. It appears that the quality and quantity of amendment supplied is a key driver of community structure, regardless of whether the amendment is from an organic or inorganic source, but the effect of carbon source and rate on the microbial community is still unclear. The next step of this project is to identify the key microbial species that are changing and their functional roles, before linking this with the changes in soil physicochemical characteristics and crop performance after subsoil manuring.
Useful resources
ULA00008 Final Report: Validating subsoil manuring in the High Rainfall Zone
Groundcover TV Episode 12: Subsoil manuring's positive impact on crop yields
References
Clark GJ, Dodgshun N, Sale PWG, Tang C (2007). Changes in chemical and biological properties of a sodic clay subsoil with addition of organic amendments. Soil Biology & Biochemistry 39, 2806–2817. doi:10.1016/j.soilbio.2007.06.003.
Gill JS, Clark GJ, Sale PWG, Peries R, Tang C (2012). Deep placement of organic amendments in dense sodic subsoil increases summer fallow efficiency and the use of deep soil water by crops. Plant and Soil 359, 57–69. doi:10.1007/s11104-012-1126-6.
Gill JS, Sale PWG, Tang C (2008). Amelioration of dense sodic subsoil using organic amendments increases wheat yield more than using gypsum in a high rainfall zone of southern Australia. Field Crops Research 107, 265–275. doi:10.1016/j.fcr.2008.02.014.
Lehman RM, Acosta-Martinez V, Buyer JS, Cambardella CA, Collins HP, Ducey TF, Halvorson JJ, Jin VL, Johnson JMF, Kremer RJ, Lundgren JG, Manter DK, Maul JE, Smith JL, Stott DE (2015). Soil biology for resilient, healthy soil. Journal of Soil and Water Conservation 70, 12A–18A. doi:10.2489/jswc.70.1.12A.
MacEwan RJ, Crawford DM, Newton PJ, Clune TS (2010). High clay contents, dense soils, and spatial variability are the principal subsoil constraints to cropping the higher rainfall land in south-eastern Australia. Australian Journal of Soil Research 48, 150. doi:10.1071/SR09076.
Murphy BW (2015). Impact of soil organic matter on soil properties - A review with emphasis on Australian soils. Soil Research 53, 605–635. doi:10.1071/SR14246.
Sale PWG, Gill JS, Peries R, Tang C (2013). Improving grain yields in the Victorian high rainfall zone with subsoil manuring. Scientific Report for GRDC Project ULA000008.
Zhang H, Turner NC, Poole ML, Simpson N (2006). Crop production in the high rainfall zones of southern Australia-potential, constraints and opportunities. Australian Journal of Experimental Agriculture 46, 1035–1049.
Acknowledgements
The scholarship for this work was provided through the GRDC and its support is gratefully acknowledged. This PhD project is funded by an Australian Postgraduate Award with top-ups generously provided by the Tim Healey Memorial Scholarship.
Contact details
Corinne Celestina, La Trobe University
celestina.c@students.latrobe.edu.au
@c_celestina
GRDC Project Code: DAV00149,
Was this page helpful?
YOUR FEEDBACK