Characterising soil borne disease risk in the eastern wheat belt of Western Australia and the national significance of major diseases
Author: Kylie Chambers, Alan McKay, Daniel Hüberli, Marg Evans, Gupta Vadakattu and Grant Hollaway | Date: 27 Feb 2018
Key Messages
- Pathogens of important diseases of cereals frequently detected in a recent survey of the eastern wheat belt of Western Australia in 2017 included:
- Crown rot (62% of samples tested)
- Root lesion nematodes (79% of samples tested)
- Rhizoctonia root rot (49% of samples tested)
- The impact of root diseases can be difficult to recognise and targeted testing of paddocks can identify situations of increasing or high risk. While pathogen levels detected by PREDICTA® B are an important indicator of potential disease risk, seasonal conditions, crop choice and management practices are also important factors in determining how much disease actually develops.
- The lack of profitable non-cereal rotation options, and the use of stubble retention to conserve moisture, can make it difficult to manage root diseases, particularly crown rot.
- While reducing inoculum levels is desirable, research in this region may need to include exploring improved non-host rotational crops and selecting well adapted cereal varieties that can perform in the presence of root disease, combined with cropping practices that reduce moisture and heat stress during grain fill.
- To identify and quantify the pathogens of soil borne diseases present in cropping paddocks in Western Australia’s eastern wheat belt (Kwinana East).
- Review data on the national impact of the major diseases and implications for the eastern wheat belt.
Aims
- To identify and quantify the pathogens of soil borne diseases present in cropping paddocks in Western Australia’s eastern wheat belt (Kwinana East).
- Review data on the national impact of the major diseases and implications for the eastern wheat belt.
Method
PREDICTA B survey
During March to June in 2017, a total of 136 soil samples (Figure 1) from 128 cropping paddocks were collected to identify and quantify soil borne disease pathogen levels using the PREDICTA B DNA based soil test. A total of 65 sample kits were sent to seven local agronomists who collected samples from cereal paddocks of growers who have not used PREDICTA B in the past. The other 71 samples were collected by DPIRD staff from paddocks selected at the grower’s discretion. Samples were collected according to the PREDICTA B sampling protocol and sent to the South Australian Research and Development Institute (SARDI) for analysis through PREDICTA B.
PREDICTA B determines the level of pathogen DNA in the soil. The results are reported as a ‘disease risk’ category for pathogens for which the relationship between pathogen DNA level and yield loss has been determined or as a ‘population density’ category for pathogens for which the DNA level/yield loss relationships are still under development.
The percentage of paddocks in the eastern wheatbelt reported as having a high, medium or low disease risk was determined for individual diseases. The percentage of paddocks in which the pathogen was below the detection limit of PREDICTA B was also identified.
For pathogens reported as population density categories, the percentage of eastern wheatbelt paddocks in which the pathogen was detected was summarised.
National root disease survey
Root disease levels were assessed within the 250 paddocks (wheat and barley) monitored by the National Paddock Survey during 2015 and 2016. Each paddock was split into two production zones. Within each production zone a transect with 5 measuring points was assessed for root disease severity by Gupta Vadakattu. A total of five plants at ZGS30 from both sides of a 0.5 m crop row at each measuring point (for both production zones) were collected for scoring. The mean root disease score was then determined for each region nationally.
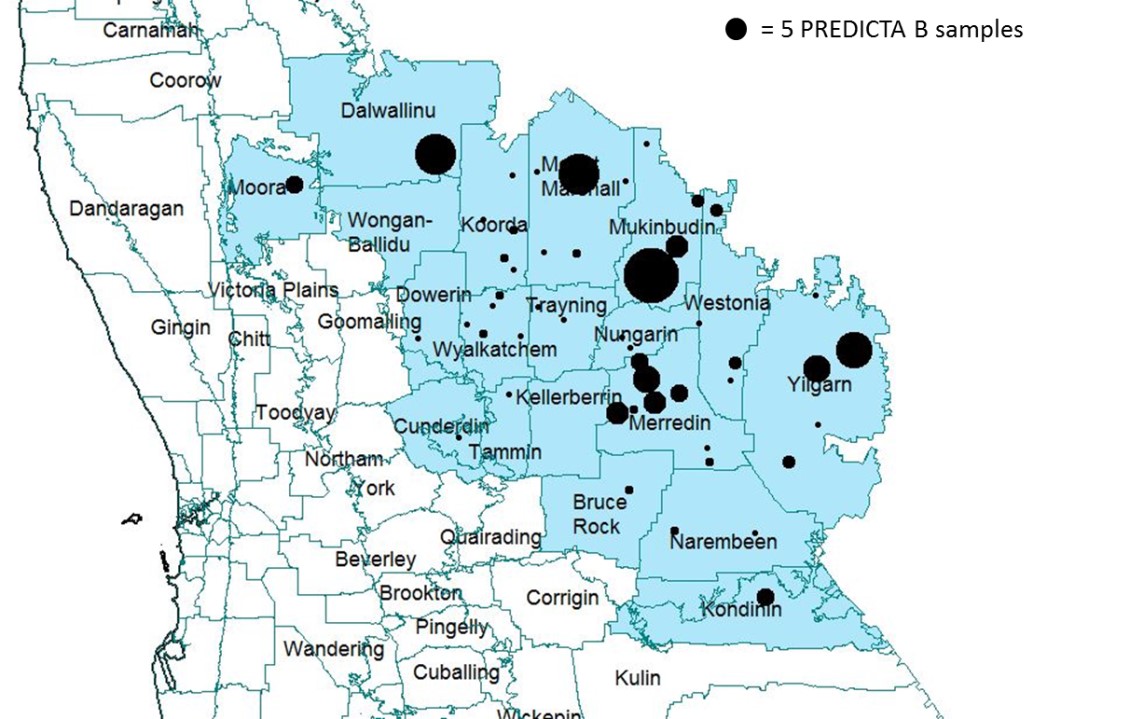
Figure 1: Distribution of PREDICTA B sampling sites in 2017 survey. Sites grouped to nearest town/ locality and circle size represents the number of samples collected from each town/ locality.
Results
Results from the survey of 128 cropping paddocks in the eastern wheat belt identified Fusarium pseudograminearum (crown rot), Rhizoctonia solani (rhizoctonia) and Pratylenchus neglectus (root lesion nematode) at medium to high levels (potential for > 10% yield loss) in soil from 44%, 57% and 20% of paddocks, respectively during 2017 (Table 1). Approximately 69% of paddocks had more than one of these three diseases. Symptoms of crown rot, rhizoctonia root rot and root lesion nematode were observed in this region during the 2017 season, including complexes of these diseases.
Gaeumannomyces graminis (take-all) was detected frequently in the PREDICTA B survey (51% of samples; Table 1), although it is thought to be of less consequence in the eastern wheatbelt due to the greater frequency of low rainfall seasons, which are less conducive to infection and spread of the pathogen within root systems.
Table 1: Summary of soil-borne disease risk in the eastern wheatbelt of Western Australia before sowing in 2017 based on 136 test results
Pathogen | Disease risk* | |||
|---|---|---|---|---|
High (%) | Medium (%) | Low (%) | Not detected (%) | |
Fusarium pseudograminerum (crown rot) | 36 | 8 | 18 | 38 |
Pratylenchus neglectus (root lesion nematode) | 5 | 52 | 22 | 21 |
Rhizoctonia solani (rhizoctonia root rot) | 4 | 16 | 29 | 51 |
Gaeumannomyces graminis (take-all) | 0 | 21 | 30 | 49 |
Didymella pinodes/ Phoma pinodella (blackspot) | 1 | 2 | 8 | 89 |
Phoma Koolunga (blackspot) | 0 | 0 | 0 | 100 |
Pratylenchus thornei | 0 | 0 | 0 | 100 |
Stem nematode | 0 | 0 | 0 | 100 |
Cereal cyst nematode | 0 | 0 | 0 | 100 |
* risk categories are a guide only, may be subject to regional and seasonal differences, and may be revised over time
Other soil-borne diseases such as Bipolaris sorokiniana (common root rot) and Pythium spp. (pythium root rot) were also frequently detected within the survey, but for these diseases risk calibrations have not been developed (Table 2). Macrophomina phaseolina (charcoal rot) was detected in all but one sample; however, charcoal rot currently appears to be ubiquitous across Western Australia and its impact has yet to be determined.
Table 2: Summary of detection of pathogens associated with the following soil-borne diseases in the eastern wheatbelt of Western Australia before sowing in 2017 (risk calibrations not yet developed for these diseases)
Pathogen (Disease) | Detection | |
|---|---|---|
Detected (%) | Not detected (%) | |
Pythium spp (pythium root rot) | 72 | 28 |
Bipoloaris sorokiniana (common root rot) | 53 | 47 |
Eutiarosporella tritici-australis (white grain) | 18 | 82 |
Pratylenchus quasitereoides (root lesion nematode) | 1.5 | 98.5 |
Pratylenchus penetrans (root lesion nematode) | 1 | 99 |
Eutiarosporella darliae/ pseudodarliae (white grain) | 0 | 100 |
Phytophthora medicaginis(phytophthora root rot- chickpeas) | 0 | 100 |
Macrophomina phaseolina (charcoal rot) | 99 | 1 |
The pathogens of several diseases were below the detection limit in the survey, including cereal cyst nematode, stem nematode, Phytophthora medicaginis (phytophthora root rot of chickpeas) , Phoma koolunga (black spot of pea), Eutiarosporella darliae/ pseudodarliae (white grain) and the nematode, Pratylenchus thornei (Tables 1 and 2).
National root disease survey
Root disease surveys of paddocks monitored by the National Paddock Survey in 2015 and 2016, show widespread moderate levels of root disease in cereal crops across Australia. Root disease ratings varied between seasons and districts (Figures 2 and 3). In general, each 0.5-unit increase in root disease score above a base of 0.5 units can reduce yield by 10%. This means that in WA the average yield losses caused by soil-borne diseases could be as high as 20% in 2015 and 40% in 2016. Final losses however depend on seasonal conditions, especially during grain fill.
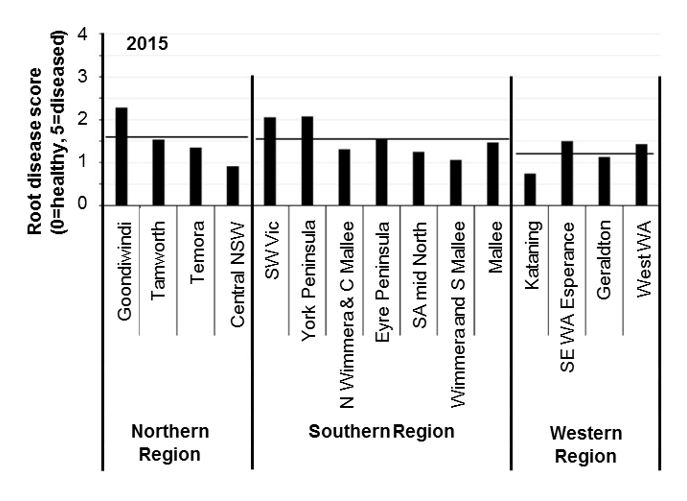
Figure 2: Average root disease ratings in cereal crops sampled 8 weeks after emergence from paddocks monitored by the National Paddock Survey project in 2015. Horizontal lines represent the average score in each region
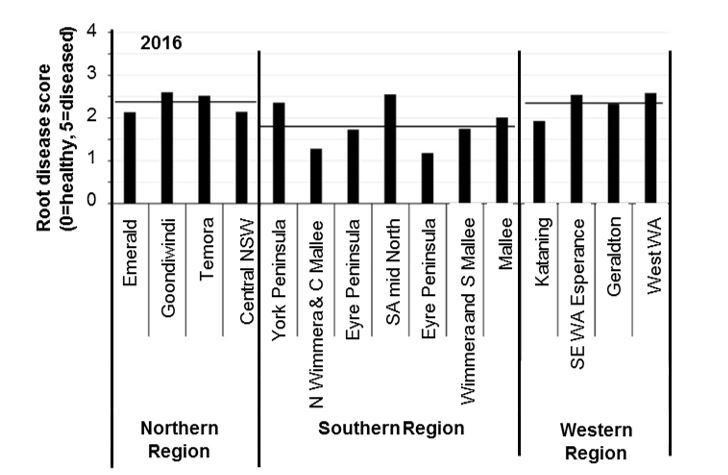
Figure 3: Average root disease ratings in cereal crops sampled 8 weeks after emergence from paddocks monitored by the National Paddock Survey project in 2016. Horizontal lines represent the average score in each region.
Impact of crown rot
Crown rot is an important disease throughout most WA cropping regions. Six wheat and barley trials conducted in Merredin (eastern wheatbelt) and Wongan Hills during 2014 to 2016 showed average yield losses of 0.37 t/ha in barley and 0.41 t/ha in bread wheat (Huberli et al. 2017). Varieties that had the lowest yield losses in these inoculated crown rot trials included Emu Rock for wheat, and Litmus and La Trobe for barley. These results are significant for the eastern wheatbelt of WA as they indicate conditions inherent to this region, particularly dry finishes to the season, which favour development of crown rot, are likely to result in significant yield losses.
Impact of rhizoctonia root rot
Rhizoctonia solani AG8, the cause of rhizoctonia root rot, is adapted to low rainfall conditions, especially in districts with sandy and non-wetting soils. It is notorious for causing distinctive bare patches that have poor yield. These patches develop when seminal roots of seedlings are severely damaged and can be worse when early root growth is restricted by cold soil temperatures, compaction layers, inadequate soil moisture below 10 cm and herbicide damage, for example.
Less well known is that crops sown early into warm moist rhizoctonia-infected soil often establish and grow well until mid-winter, then when soil temperatures drop to around 10°C or lower, rhizoctonia root rot infects the crown roots that support tillering causing the crop to develop uneven growth. Barley is more vulnerable than wheat because it produces more tillers and therefore, is more dependent on crown roots.
Yield losses can exceed 50% in extreme circumstances, but this can vary between seasons. The symptoms are often most visible in low rainfall seasons; however, research supported by GRDC, SAGIT, DPIRD, UniSA and SARDI in low rainfall areas of SA and WA have shown a strong effect of growing season rainfall on the magnitude of yield response with lower rainfall conditions having a lower yield response.
So yield losses in the eastern wheatbelt of WA caused by rhizoctonia root rot alone may be significant but not as great as the crop symptoms might suggest in low rainfall seasons.
Impact of Pratylenchus neglectus
Field experiments conducted in Western Australia, South Australia and Victoria since 2010 have shown that yield loss caused by P. neglectus is related to their concentration in the soil at planting, but the extent of the yield loss is dependent on seasonal conditions and cultivar grown. Rotations that reduce nematode numbers provide a means for reducing losses. Trials from Western Australia have also shown that the major break crop, canola, does not reduce nematode numbers and can also exhibit yield losses from P. neglectus, with medium-high levels of the nematode causing an average yield loss of 16% (Collins et al. 2017).
Lupins have been shown to reduce P. neglecutus populations and can be a good choice to manage RLN (Collins et al. 2017), however, lupins only occupy a small percentage of land use in the eastern wheat belt. Further work is required to understand the impacts of P. neglectus on varieties and identify alternative profitable break crops or pastures to help manage this disease in the eastern wheatbelt.
Conclusion
Our 2017 pre-sowing survey of soil borne diseases in WA’s eastern wheat belt established that 99% of paddocks tested had one or more pathogen present. The pathogens that cause crown rot, rhizoctonia root rot and root lesion nematode were most commonly detected in the survey paddocks. These pathogens are capable of causing significant yield loss if suitable conditions occur. Also, symptoms of all three diseases were observed during the 2017 growing season.
It can be difficult to visually recognise root disease impacts in crops so targeted testing of paddocks using PREDICTA B or other diagnostic services can help identify situations of increasing or high risk. The conversion of these risks to actual impacts will vary from season to season depending on seasonal conditions, crop choice and management practices. For example, yield losses from soil borne diseases and nematode pests may be minimised when moisture stress is low during flowering and grain fill.
The eastern wheatbelt is unique and currently has limited profitable non-cereal rotation options, so break crops to manage the inoculum levels of crown rot, rhizoctonia root rot or root lesion nematodes in this growing zone are not always a simple solution. Research that targets the particular characteristics of the region is necessary. For instance, investigation of well adapted crops/varieties with useful levels of disease resistance against these major pathogens is important. As is optimising cropping practices, such as sowing times to minimise the impact of moisture and heat stress during grain fill, inter-row sowing to limit seedling proximity to inoculum and crop nutrition to avoid exacerbating disease expression and maximise profitability.
The impact and management of root disease complexes including different combination of crown rot, rhizoctonia and root lesion nematodes also needs to be investigated as there is evidence that yield losses caused by disease complexes are greater than the combined losses of each individual disease.
Useful PREDICTA B links
- Current tests: SARDI Molecular Diagnostics
- 2017 PREDICTA B maps
- Download the latest consultant resource manual
References
Collins S, Wilkinson C, Kelly S, Hunter H, DeBrincat L, Reeves K, Chen K (2017). The invisible threat: Canola yield losses caused by root lesion nematodes in WA. In: 2017 Grains Research Updates, Perth, Western Australia, 27 – 28 February
Hüberli D, Gajda K, Connor M, Van Burgel A (2017). Choosing the best yielding wheat and barley variety under high crown rot. In: 2017 Grains Research Updates, Perth, Western Australia, 27 - 28 February
Acknowledgments
Surveys supported through DAV00128 (National Nematology project) by GRDC and Agriculture Victoria.
This project also supported by GRDC, DPIRD and Royalties for Regions investment through projects: DAS00137 (National molecular diagnostics project), DAN00175 (National crown rot project), BWD000025 (National Paddock Survey project) funded by GRDC and DAW00256 (Building crop protection and production agronomy R&D capacity in regional Western Australia project).
Thank you to Jenni Clausen, Stacey Hansch, Bec Swift, Geoff Thomas, (DPIRD), Cam Smith, Sophie Hooper (Elders), Matt Willis (then Elders), James Lydon, Benita Moir, Darren Marquis (Landmark) and Jessica Smith (DKT and Landmark) for sample collection.
I also thank the growers who participated in this survey. Thank you to Geoff Thomas (DPIRD) for reviewing this paper.
GRDC Project Number: DAV00128, DAS00137, DAS00125, DAN00175, BWD000025 and DAW00256
Varieties displaying this symbol beside them are protected under the Plant Breeders Rights Act 1994.
GRDC Project Code: DAV00128, DAS00137, DAS00125, DAN00175, BWD000025 and DAW00256,
Was this page helpful?
YOUR FEEDBACK
