Physiological and biochemical responses of lentils to silicon mediated drought tolerance
Author: Sajitha Biju, Sigfredo Fuentes, Dorin Gupta (School of Agriculture and Food, Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Parkville, VIC). | Date: 27 Feb 2018
Take home messages
- Silicon (Si) improves seed germination and alleviates drought stress in lentil crops by regulating osmolytes, hydrolytic enzymes and antioxidant defence systems.
- Silicon potentiates photosynthetic efficiency and biochemical defence responses of lentils against drought stress.
- Silicon improves the yield traits in drought stressed lentils.
Background
This project, through investment by GRDC, is focused on elucidating the role of silicon (Si) in conferring drought tolerance in lentils, an important legume crop of Australia. The project investigates the possible physiological, biochemical and molecular mechanisms behind SI mediated drought tolerance in lentils. We have identified drought tolerant genotypes through a screening experiment in glasshouse studies. Another glasshouse experiment was carried out to investigate the role of Si application and drought stress in lentil genotypes.
Results from the experiments conducted so far are promising. They revealed that the yield traits, photosynthetic efficiency, the concentration of reactive oxygen species (superoxide radicals and hydrogen peroxide (H2O2), and the antioxidant compounds and enzymes in glutathione ascorbate (GSH-AsA) cycle, which were the main factors related to reduced growth and yield in response to drought, increased significantly with Si application under drought stress. Thus, Si could ameliorate adverse effects of drought stress in lentil crops likely by increasing photosynthetic efficiency, reducing oxidative stress and osmotic stress.
The results from these experiments would be further validated by field trials and other relevant molecular experiments in the laboratory at The University of Melbourne (UM).
Introduction
Lentils (Lens culinaris Medik) are the most ancient cultivated crop among legumes and an important source of protein, minerals and vitamins for the human diet (Yadav et al. 2007). Lentils are classified as a Si excluder and are moderately tolerant to drought stress. Even though lentils are a moderately drought tolerant crop and can grow in reduced water supply, plant productivity can decrease from 6% to 54% under a range of drought stress conditions (Siddique et al. 1999).
Severe water stress can lead to total crop failure, especially in semi-arid regions, where they are commonly exposed to intermittent or terminal drought stress conditions. Lentils are highly sensitive to drought stress at key growth stages, such as seedling, flowering and grain filling (Shrestha et al. 2006). With the forecast of increased water scarcity in the near future, drought stress will remain a major threat to global lentil production. Breeding for drought tolerance remains challenging due to variation in climatic conditions and multigenic origin of the adaptive responses of lentil plants to drought stress (Kumar et al. 2016; Idrissi et al. 2016). Therefore, another more sustainable approach is the use of exogenous compounds, which are easily available and cost effective to incorporate into agronomical practices with negligible detrimental effects to the environment.
Silicon (Si) is the second most ubiquitous element in the earth’s crust which has been shown to be effective in improving drought tolerance in some Si-accumulating monocot plants such as rice (Chen et al. 2011), sorghum (Hattori et al. 2007), maize (Sayed and Gadallah, 2014) and wheat (Pei et al. 2010). It has also been shown to be effective in a few dicot plants such as sunflowers (Gunes et al. 2008), cucumbers (Ma et al. 2004), soybeans (Shen et al. 2010), tomatoes (Shi et al. 2016) and chickpeas (Kurdali et al. 2013).
Priming of seeds with S has been considered as one of the alternative methods to improve drought tolerance in plants (Hameed et al. 2013; Ahmed et al 2016). Si-seed may fortify plants against future stress events and appears to be a promising and cost-effective procedure. Under drought stress conditions, Si is thought to act as a mechanical barrier to minimise transpiration losses and also mediates many metabolic, physiological and biochemical pathways which subsequently improve drought tolerance.
A number of possible mechanisms were proposed through which Si may increase drought tolerance in plants, especially improving the water status of plants, increased photosynthetic activity and ultra-structure of leaf organelles (Coskun et al. 2016). However, most of the studies are carried out in monocots which tend to be high Si accumulators and only little research has been conducted in dicot plants, which usually have low capability of Si accumulation. Thus, studying the role of Si in low Si-accumulating legumes (Meena et al. 2014) such as lentils would help to unravel the actual mechanism and function of Si-mediated drought tolerance in plants and the ability of lentil crops to cope with drought stress conditions with a minimum impact on critical growth stages.
Germination and seedling development are very important stages that determine the successful early establishment of a plant in its growing environment. Water plays a key role in germinating seeds through hydrolytic breakdown of food reserves, solubilisation and the transport of metabolites, osmotic adjustment and enzymatic reactions. However, water scarcity seriously hampers successful seedling establishment (Shi et al. 2016).
Hydrolytic enzymes such as α-amylase, β-amylase and α-glucosidase play a pivotal role during seed germination by hydrolysing starch into sugar. The activity of these enzymes is suppressed by drought stress with negative impacts on carbohydrate metabolism. Accumulation of the osmolytes (proline, glycine betaine-GB and soluble sugar) under drought stress in many plants has been positively correlated with water stress tolerance. These compounds are thought to play adaptive roles in mediating osmotic adjustment and protecting subcellular structures in stressed plants (Ashraf & Foolad, 2007; Singh et al. 2015; Blum et al. 2017).
Similarly, it is well known that abiotic stresses including drought stress can cause oxidative damage to plants, either directly or indirectly through the formation of reactive oxygen species (ROS), such as superoxide anion- O2.− and H2O2 (McCord, 2000; Das & Choudhary, 2016). Plants respond to this oxidative stress by increasing the production of antioxidant enzymes such as ascorbate peroxidase (APX: EC.1.11.1.11), catalase (CAT, EC 1.11.1.6) and superoxide dismutase (SOD, EC 1.15.1.1), which can scavenge ROS and in turn protect the plants from oxidative damage (Noctor et al. 2014; Das & Roychoudhary, 2016).
Since their activities and transcripts are altered when plants are subjected to stress conditions, changes in the levels of antioxidant enzymes and ROS have been used to assess the effect of drought stress in plants (Hasegawa et al. 2000; Hernandez et al. 2000). Enhanced germination of seeds by Si application has been shown in different monocots and dicots under controlled conditions (Torabi et al. 2012). Relatively few studies have investigated the effect of Si on seed germination rate and seedling growth under drought stress in plants such as wheat and tomatoes (Hameed et al. 2013; Shi et al. 2014). However, the effect of Si on the concentrations of osmolytes activity of hydrolytic enzymes, activity of antioxidant enzymes, the concentration ROS and the level of lipid peroxides (LPX) under drought stress has not been investigated so far in seed germination studies of crop plants. Moreover, to the best of our knowledge, no studies have been undertaken until now to elucidate the role of Si in drought stressed lentil plants.
Therefore, given the potential of Si for drought tolerance (Liang et al. 2003; Coskun et al. 2016) and lentils being one of the most nutritious plants of the future, the objectives of the present study were specifically (1) to elucidate the role of Si in stimulating seed germination by evaluating the metabolism of osmolytes, antioxidants and the activity of hydrolytic enzymes under controlled and drought stress conditions, and (2) to recommend Si as a source to improve drought stress tolerance in lentil crops.
Materials and methods
Plant materials, germination conditions and experimental design
Seeds from seven lentil genotypes i) ILL 6002 and ii) Indianhead (drought-tolerant); iii) PBA Flash, iv) PBA Jumbo 2 and v) Nipper (moderately drought tolerant); vi) PI 468898 and vii) ILL 1796, (drought sensitive) were procured from The Australian Grains Genebank (AGG), Horsham, Victoria(VIC) (Table 1). Among the genotypes, PBA Jumbo 2 is the newly released and highest yielding lentil variety in Australia, outperforming all other current varieties in medium-rainfall lentil growing regions (www.pbseeds.com.au).
Furthermore, ILL 6002 is a potential drought tolerant genotype with a well developed root system and high early biomass, which has been widely used in many breeding programs (Sarker et al. 2005; Kumar et al. 2016) and also for drought-related quantitative trait loci (QTL) mapping (Idrissi et al. 2016). The rest of the genotypes used in this study were selected and categorised based on a drought tolerance screening study of 37 lentil genotypes using infra-red thermal imaging, growth and productivity traits analysed using multivariate data analysis (under publication).
Table 1. Lentil genotypes and their abbreviations used in the different drought-stress treatments such as control (C), drought stress (D), drought stress + Si (DSi) and Si alone (Si).
Genotypes and colour code | Drought-tolerance | Abbreviations | |
|---|---|---|---|
1 | ILL 6002 | Tolerant | G1 |
2 | Indianhead | Tolerant | G2 |
3 | PBA Jumbo 2 | Moderately tolerant | G3 |
4 | Nipper | Moderately tolerant | G4 |
5 | Flash | Moderately tolerant | G5 |
6 | PI 468898 | Drought-sensitive | G6 |
7 | ILL 1796 | Drought-sensitive | G7 |
The seeds were selected by size homogeneity and were surface-sterilised for 20 minutes in 30% (v/v) H2O2, rinsed and soaked in distilled water for 1 hour. For each of the seven genotypes, five replicates of 10 seeds were placed on a filter paper in 9cm diameter petri dishes containing 3cm3 of distilled water (control-C), 18% of polyethylene glycol-6000 (PEG, MW 6000) concentration corresponding to final osmotic potentials of −0.58 MPa (Muscolo et al. 2014) (drought stress-D), 18% PEG with 2mM Si (drought stress with supplemented Si-DSi) and Si alone (Si).
PEG is most commonly used to induce osmotic stress in plants. PEG is used to induce the osmotic stress or water-deficit condition because it is not naturally produced in plant tissue and cannot penetrate into cells from the media. PEG induced stress can eventually destroy the normal emergence, growth, biochemical attributes and yield of wheat (Pei et al. 2010). A preliminary experiment was conducted with 0%, 15%, 18% and 21% of PEG (MW 6000) concentration corresponding to final osmotic potentials of 0, -0.30, -0.51, -0.58, and -0.80 MPa, respectively (Muscolo et al. 2014).
Based on the results, 18% PEG concentration was found to induce moderate drought stress in the studied lentil genotypes. The source of Si was sodium metasilicate (Na2SiO3). The silicate supply generally causes medium basicity due to the capability of silicate ions (SiO32−) to protonate. Therefore, sodium (Na) was replenished with sodium sulfate (Na2SO4) in control to minimise the medium basification, as well as Na effects originating from addition of Na2SiO3.
A preliminary experiment using different Si concentrations (0, 0.5, 1.0, 1.5, 2.0, 2.5, 3mM) showed that 2mM of Si had the best effect on improving the negative influence of drought stress. Therefore, the experiment was designed with 2mM of Si. The petri dishes were sealed with parafilm to prevent evaporation and kept accordingly to a completely randomised block design in a growth chamber at a temperature of 25 ± 1 °C in dark conditions with a relative humidity (RH) of 70%.
Traits used to assess germination
Seeds were considered to be germinated when the radicle extended for at least 2mM. After four days of germination, germination percentage (GP), germination index (GI), and seedling vigour index (SVI) (Yan, 2016) were measured using the following equations:
GP=n/N *100
Equation 1, where, n is the number of germinated seeds and N represents the total number of seeds tested.
GI=Σ(Gt/Dt)
Equation 2, where, Gt is the number of seeds germinated at t day and Dt represents the corresponding day of germination.
SVI=GP * mean of seedling length
Equation 3
The fresh and dry weights of the seedlings were measured before and after oven drying each sample at 80°C for 48 hrs, respectively (Yan, 2016).
Determination of proline
Free proline levels were determined by using the method proposed by Bates et al. (1973). Finely ground lentil seedlings (0.1g) were homogenised in 10mL of 3% aqueous sulfosalicylic acid (C7H6O6S) and then the homogenate was filtered through Whatman no. 2 filter paper (GE Healthcare, NSW). Two ml filtrate was reacted with 2ml acid-ninhydrin (C9H6O4) and 2mL of glacial acetic acid (C2H4O2) in a 10mL test tube for 1hr at 1000C, and the reaction was terminated in an ice-bath. The reaction mixture was extracted with 4mL toluene (C6H5-CH3), mixed vigorously with a test tube stirrer for 15–20 s. After 1h, toluene (C6H5-CH3) was added and absorbance at 520nm was measured by using a single beam scanning UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK). The standard curve for proline was prepared by dissolving proline in 3% sulfosalicylic acid (C7H6O6S) to cover the concentration range 0.5–10μg. mL-1. The proline concentration of the extract was determined from the standard curve and calculated on a fresh weight basis as follows:
[(μg proline/ml * X ml toluene)/115.5μg/μmol]/ [(g sample)/5] = μmol proline/g fresh weight sample
Equation 4, where, X= ml of toluene used to extract the reaction mixture (4ml).
Determination of glycine betaine (GB)
Concentration of GB was estimated from finely ground lentil seedlings (500mg), which was produced by mechanical shaking with 20mL deionised water for 24h at 25°C. Extracts were filtered and subsequently diluted (1:1) using sulphuric acid (H2SO4) (2 N), with 0.5mL of extract cooled in an ice bath for 1h, after which 0.20mL cold potassium iodide (KI–I2) reagent was added.
The resultant solution was stirred and kept for 16h at 4°C, then centrifuged at 10,000 rpm for 15 minutes. Thereafter, the supernatant was carefully aspirated with a fine-tipped glass tube and 1, 2-dichloroethane was added to dissolve the periodide crystals; absorbance was measured at 365nm using a single beam scanning UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK). The standard reference for GB was used for calculation (Grieve and Grattan, 1983).
Determination of total soluble sugar
Total soluble sugar content was determined by the anthrone method (Dubois et al. 1951), a 100mg sample was homogenised with 5mL 80% ethanol (C2H5OH). The extract was centrifuged at 4000rpm for 15 minutes. The supernatant was separated, with the remaining residue being extracted twice using 5ml of 80% ethanol. Supernatant was collected in the same flask and total volume was maintained to 15mL with 80% ethanol. The residue was then used for the extraction and estimation of total soluble sugar. An extract volume of 0.1mL was taken in a 10mL test tube and oven dried at 60°C. The final volume was made to 1.0mL using distilled water. A volume of 5mL anthrone reagent (0.2% anthrone in concentrated H2SO4 was added. All tubes were placed in a boiling water bath for 10 minutes and were cooled immediately in cold running water. A blank was prepared in a similar way by taking 1.0mL distilled water. The absorbance was measured at 620nm with a single beam scanning UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK). The amount of sugar from the samples was calculated by the standard curve (Dubois et al. 1951) and expressed asmg. g-1 fresh weight.
Assay of hydrolytic enzymes
The activities of the hydrolytic enzymes, α-amylase (E.C. 3.2.1.1), β-amylase (E.C. 3.2.1.2) and α-glucosidase (E.C. 3.2.1.21) were determined from the crude extracts of each lentil genotype. For this purpose, the seedlings of each genotype were homogenised in a chilled mortar with distilled water 1:4 (w/v) and centrifuged at 14,000rpm for 30 minutes. The supernatant was filtered through a single layer of muslin cloth and was used for α-amylase (Steup, 1988), β-amylase (Steup, 1988), and α-glucosidase (Bergmeyer et al. 1983) activity determination. For α -amylase, a mixture of 3mL soluble starch (2% v/v) and 3mL extract was incubated for 60 minutes at 30°C. After incubation, an equal volume of alkaline colour reagent was added to a 1mL incubation mixture, was and was then mixed and heated for five minutes in a boiling water bath.
The absorbance at 546nm was measured against a blank (1mL distilled water + 1mL alkaline reagent) using a single beam scanning UV/visible spectrophotometer. The standard curve was obtained by measuring the absorbance from the spectrophotometer of different known maltose concentrations in the range of 0-2.5mM. L-1.
The alkaline colour reagent was prepared by dissolving 1g of 3, 5-dinitrosalycylic acid (C7H4N2O7) in a mixture of 40mL 1 N sodium hydroxide (NaOH) solution and 30mL of distilled water. Twelve grams of solid potassium sodium tartrate (KNaC4H4O6.4H2O) was added and dissolved. The mixture was brought to a final volume of 100mL using distilled water. β-amylase activity was determined as described above but soluble starch was replaced by amylopectin.
For α-glucosidase detection, the assay buffer was prepared by using 50mM sodium acetate (C2H3NaO2) and 10mM calcium chloride (CaCl2) and the final pH was adjusted to 5.2. The substrate was 10mM. L-1 maltose. The samples were incubated for 60 minutes. The release of glucose was measured by changes in nicotinamide adenine dinucleotide phosphate (NADPH) at 340nm absorption using the single beam scanning UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK) in a coupled enzyme reaction of hexokinase (EC 2.7.1.1) and glucose-6-P dehydrogenase (EC 1.1.1.49).
Extraction and assay of antioxidant enzymes and reactive oxygen species (ROS)
For extraction of the antioxidant enzymes, lentil seedlings were initially ground to powder in liquid nitrogen and then extracted with 50mM sodium phosphate buffer (pH 6.8) for peroxidase (POX) and catalase (CAT) and 50mM sodium phosphate buffer (pH 7.2) for ascorbate peroxidase (APX) and 100mM potassium phosphate buffer (pH 7.6) for superoxide dismutase (SOD) using polyvinyl pyrrolidone under ice cold conditions. The homogenates were then centrifuged at 10,000rpm for 10 min at 48°C. Supernatants were used as crude enzyme extracts. Protein content in each of the extract was estimated following the method of Lowry et al. (1951) using bovine serum albumin (BSA) as standard curve.
Ascorbate peroxidase (APX: EC.1.11.1.11)
APX activity was assayed as decrease in absorbance by monitoring the oxidation of ascorbate at 290nm with a UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK) according to the method of Chen and Asada (1989). The reaction mixture consisted of 0.01mL of enzyme extract, 0.5mM ascorbic acid (ASC), 30% H2O2 (v/v) and 0.05M sodium phosphate buffer (pH 7.2). The molar absorption coefficient of ASC (2.8mM.cm−1) was used to calculate the enzyme activity. Enzyme activity was finally expressed asmMol ascorbate.mg-1 protein. min-1.
Peroxidase (POX: EC 1.11.1)
For determination of POX activity, 40µL of freshly prepared crude enzyme extract was added to the reaction mixture containing 1ml of 0.1M phosphate buffer (pH 6.8), 1mL of 20mM guaiacol and 50µL of 10mM H2O2. POX activity was assayed spectrophotometrically in a UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK) at 470nm for 10 min at 30—C 460nm by monitoring the oxidation of guaiacol in the presence of H2O2 (Goliber, 1989). Specific activity was expressed asmMol guaiacol.mg-1 protein. min-1.
Catalase (CAT: EC.1.11.1.6)
The CAT activity was assayed as described by Chance and Maehly (1955). Enzyme extract (40µL) was added to 30% H2O2 (v/v) in phosphate buffer pH 6.8 and the breakdown of H2O2 was measured at 240nm in a UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK). An equivalent amount of buffer containing H2O2 was used as reference. The enzyme activity was expressed asmMol H2O2mg-1 protein. min-1. The molar absorption coefficient of H2O2 (0.04mM-1. Cm-1) was used to calculate the enzyme activity.
Superoxide dismutase (SOD: EC 1.15.1.1)
The SOD activity was assayed by monitoring the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT) according to the method of Dhindsa et al. (1981). Three ml of the assay mixture constituted of 0.1ml enzyme extract, 100mM phosphate buffer (pH 7.6), 1.5mM Na2CO3, 2.25mM NBT, 200mM methionine, 3mM ethylene diamine tetra acetic acid (EDTA), 0.06mM riboflavin and distilled water. The reaction tubes containing enzyme samples were illuminated with a 15 W fluorescent lamp for 10 min. The other set of tubes lacking enzymes were also illuminated and served as a control. A non-irradiated complete reaction mixture served as a blank. The absorbance of samples was measured at 560nm with a UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK) and 1 unit of activity was defined as the amount of enzyme required to inhibit 50% of the NBT reduction rate in the controls containing no enzymes.
Quantification of superoxide anion (O2−)
The O2.− concentration was quantified, following the method of Doke (1983). One gram tissue was homogenised in a pre-chilled mortar using 50mM sodium acetate buffer containing 10mM sodium chloride (NaCl) (pH 6.5). The homogenate was filtered by double-layered cheesecloth and centrifuged at 10000rpm for 10 min. The supernatant was collected and made up to a known volume. An aliquot (0.1ml) of the extract was taken and mixed with the assay reagent containing 0.01M potassium phosphate buffer (pH 7.8) with 0.05% nitro blue tetrazolium sodium salt (NBT) and 10mM sodium azide (NaN3). The assay mixture was incubated for 30 min and the initial absorbance at 580nm was taken in a UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK). Final absorbance was taken after heating the mixture at 85°C for 15 min.
Quantification of H2O2
The H2O2 concentration in the incubation medium of the treated plant tissues was estimated as per the procedure of Bellincampi et al. (2000). It was based on the peroxidase mediated oxidation of ferrous ion (Fe2+) followed by the reaction of ferric ion (Fe3+) with xylenol orange. One gram of seedling sample was taken and homogenised in 10ml of cold 10mM phosphate buffer (pH 7) using a pre-chilled mortar and pestle. The homogenate was filtered and centrifuged at 10000 rpm for 10 min. The supernatant was collected and made up to a known volume using the buffer. The extract (1.5ml) was added to an equal volume of assay reagent containing 500mM ammonium ferrous sulphate, 50mM H2SO4, 200mM xylenol orange and 200mM sorbitol. The assay mixture was incubated for 45 min and the absorbance of the Fe3+ xylenol orange complex at 560nm with a UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK) was observed.
Determination of LPX
The level of lipid peroxidation (LPX) was measured in terms of malondialdehyde (MDA) content (Heath and Packer, 1968). The plant sample (0.5g) was homogenised in 10mL 0.1% trichloro acetic acid (TCA) using a mortar and pestle. The homogenate was centrifuged at 15000 rpm for 5 min. To 1.0mL supernatant, 4.0mL 0.5% thiobarbituric acid (TBA) in 20% TCA was added. The mixture was heated at 95°C for 30 min, and then quickly cooled in an ice bath. After centrifugation at 10000 rpm for 10 min, the absorbance of supernatant was recorded at 532nm with a UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK). The value for nonspecific absorption at 600nm was subtracted. The concentration of lipid peroxides was quantified in terms of MDA level using an extinction coefficient of 155mM. cm−1 and expressed as nmol g−1 fresh weight.
Estimation of Si
Si content was determined by a modified autoclave-induced digestion (AID) method (Elliot and Snyder, 1991). The seedlings were oven dried and ground in a Retsch centrifugal mill ZM-200 (Retsch, Haan, Germany) into fine powder. The ground samples (0.1g) were wetted with 3ml of a 30% hydrogen peroxide solution and 3.25mL of 50% sodium hydroxide (NaOH) in polyethylene tubes and gently mixed by vortex. The tubes were placed in an autoclave (SABAC, model T62) at 138 kPa for 1 h at 126oC. Digested samples were brought to 50ml with distilled water. Si concentration was determined by the colorimetric molybdenum blue method (Liang et al. 2003). To 1.0mL of digested sample, 9mL of 20% acetic acid and 2.5mL ammonium molybdate (54 g.L-1, pH 7.5) was added in 50mL of polypropylene volumetric flask. Then 1.25mL of 20% tartaric acid and 0.25mL reducing solution containing 8g.L-1 sodium sulphite (Na2SO3), 1.6g.L-1 1-amino- 2-naphthol-4-sulfonic acid and 100g.L-1 sodium bisulphite (NaHSO3) were added immediately. After 30 minutes, the absorbance was measured at 650nm wavelength with a UV/visible spectrophotometer (M501, Campspec Ltd, Cambridge, UK). A standard curve was prepared from Si standard solutions (Si1000, Kanto Chemical Co. Inc., Japan).
Statistical analysis
All the experiments described were performed in triplicate. The data were analysed statistically using analysis of variance (ANOVA), followed by a tukey pairwise comparison test for mean comparison between genotypes and treatments using Minitab®v17 (Minitab Inc., Pennsylvania, USA). Significant differences among genotypes and treatments were considered at p ≤ 0.05. The data were also analysed using a multivariate analysis method based on principal component analysis (PCA), cluster analysis and covariance matrix algorithms through a customised code written in Matlab ver2016b (Mathworks Inc., Natick, MA, USA) to determine changes in drought tolerance level by Si application for the studied lentil genotypes and to determine the relationships between drought tolerance assessment traits.
Results
Effect of Si on the germination traits assessed by GP, GI and SVI in lentil genotypes
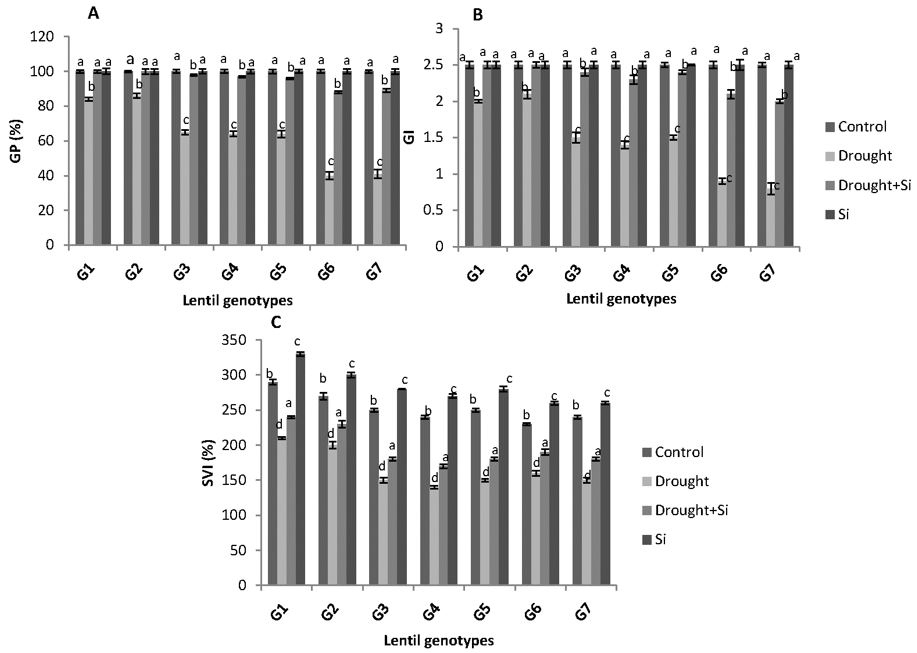
The effects of drought stress and Si treatment on the germination percentage (GP) of seven lentil genotypes, calculated using the Equation 1, are shown in Figures 1 and 2a. PEG 6000-stress significantly reduced the GP of each genotype as compared to the control. However, Si application under drought stress increased GP significantly in all of the genotypes (p ˂0.01), when compared to drought stressed plants. The effect of Si under drought stress was more pronounced in both the drought-sensitive genotypes, G6 (PI 468898) and G7 (ILL 1796) (2.5-fold increase in GP). Interestingly, irrespective of the drought tolerance capacity, all the studied genotypes exhibited 100% GP by added Si under non-stress conditions.
The GI and SVI values were calculated using Equations 2 and 3. Similar to GP values, Si significantly increased GI and SVI values among all the genotypes under drought stress (Figures 2b and 2c). The Si application did not show any significant effect on the GI of each genotype under non-stress conditions. However, Si application significantly affected the SVI values in all the studied genotypes under drought stress conditions.
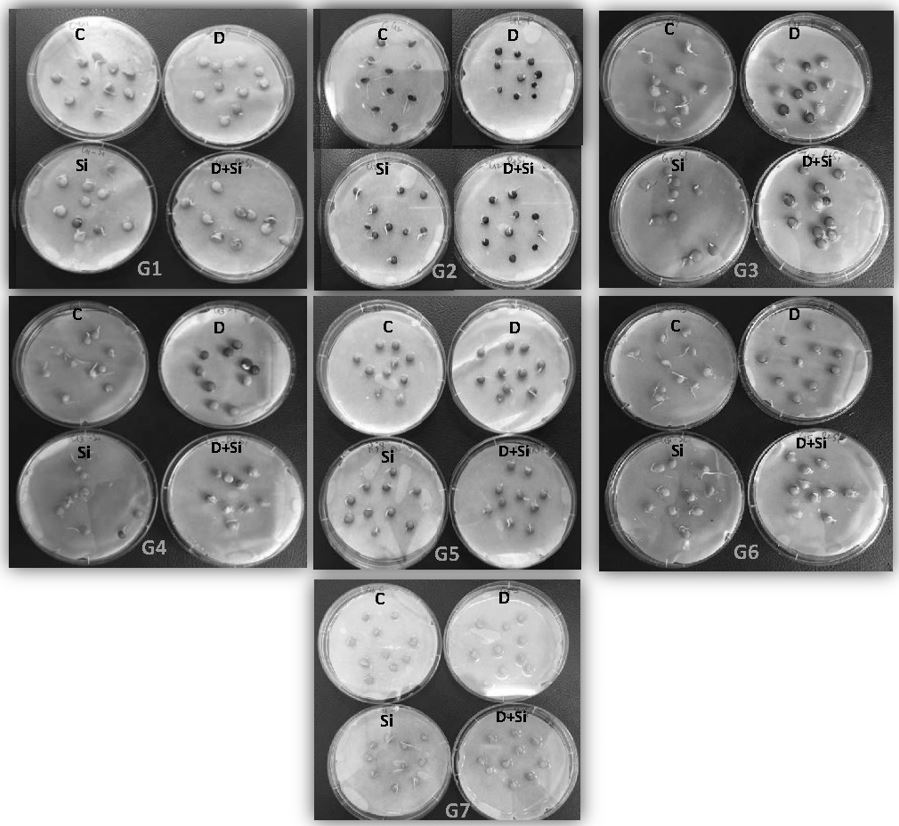
Figure 1. Seed germination of seven lentil genotypes under different drought stress treatments. ILL 6002 (G1), Indianhead (G2), PBA Jumbo 2 (G3), Nipper (G4), PBA Flash (G5), PI 468898 (G6) and ILL 1796 (G7) represent different lentil genotypes. Control (C), drought stress (D), drought stress + Si (DSi) and Si alone (Si) are the different drought stress treatments. Different letters denote statistical differences at p ˂ 0.01 within genotypes.
Figure 2a, b and c. Germination percentage (GP %), germination index (GI) and seedling vigour index (SVI-%) of the seven lentil genotypes under different drought stress treatments. ILL 6002 (G1), Indianhead (G2), PBA Jumbo 2 (G3), Nipper (G4), PBA Flash(G5), PI 468898 (G6) and ILL 1796 (G7) represent different lentil genotypes. Control (C), drought stress (D), drought stress + Si (DSi) and Si alone (Si) are the different drought stress treatments. Mean values provided with error bars represent the standard error. Different letters denote statistical differences at p ˂ 0.05 within genotypes.
Effects of Si application on fresh and dry weight of the seedlings
The fresh and dry weight of the seedlings decreased significantly with exposure to drought stress (Table 2). However, application of Si increased the fresh and dry weight under stress and non-stress conditions.
Table 2. Fresh and dry weight (mg) of the seedlings of seven lentil genotypes under different drought-stress treatments. Mean values provided with error bars representing the standard error. Different letters denote statistical differences at p ˂ 0.01 within genotypes.
Fresh Weight (mg) | Dry Weight (mg) | |||||||
|---|---|---|---|---|---|---|---|---|
Lentil | Control (C) | Drought (D) | Drought +Si (DSi) | Si (Si) | Control (C) | Drought (D) | Drought + Si (DSi) | Si (Si) |
G1 | 120±0.002b | 80±0.001d | 90±0.001c | 130±0.001a | 9±0.0001b | 4±0.0001c | 9±0.0002b | 16±0.0001a |
G2 | 80±0.001b | 50±0.002d | 70±0.002c | 140±0.002a | 5±0.0001b | 3±0.0001c | 6±0.0002b | 17±0.0002a |
G3 | 120±0.001b | 70±0.001c | 90±0.003c | 160±0.001a | 9±0.0001b | 0 | 7±0.0002c | 8±0.0002a |
G4 | 90±0.002b | 70±0.003c | 80±0.001c | 150±0.002a | 5±0.0001b | 0 | 4±0.0001c | 7±0.0001a |
G5 | 120±0.001b | 90±0.002c | 100±0.002c | 150±0.010a | 7±0.0001b | 0 | 5±0.0001c | 9±0.0001a |
G6 | 180±0.003b | 80±0.002d | 110±0.001c | 190±0.001a | 1±0.0001b | 0 | 2±0.0002b | 6±0.0002a |
G7 | 50±0.001b | 100±0.001d | 40±0.002c | 80±0.001a | 1±0.0002b | 0 | 2±0.0001b | 4±0.0002a |
Effects of Si application on the levels of osmolytes in seedlings of lentil plants
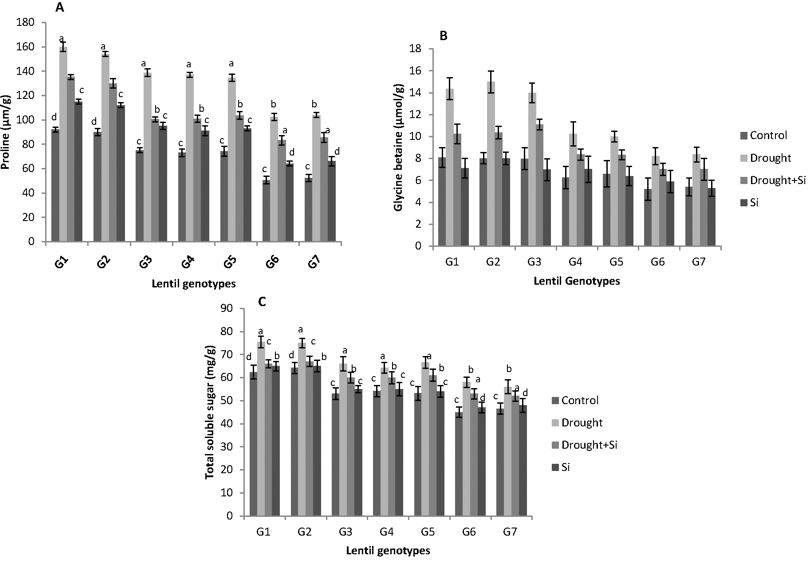
The concentrations of the osmolytes (proline, GB and total soluble sugar) significantly increased in all the lentil genotypes under drought stress, while Si treatment led to a reduction in their values (Figures 3a, 3b and 3c). Figure 3a shows the variation in proline concentration, obtained using the Eq. 4, in all the lentil genotypes under different drought stress treatments. Drought stress alone increased the proline level almost 2-fold compared to the control treatment in all the genotypes. Interestingly, proline concentration was found to be higher in drought tolerant genotypes, G1 (ILL 6002) and G2 (Indianhead), when compared to the drought-sensitive genotypes, G6 (PI 468898) and G7 (ILL 1796) in all the treatments.
The application of Si lowered the proline level of drought stressed seedlings by 20% to 25 %. Even though GB accumulation increased significantly in drought stressed seedlings, Si application resulted in 20% to 40% reduction in GB content of drought stressed plants. However, there was no significant difference in GB content of Si treated lentil seedlings under non-stress condition, compared to the control. The accumulation of GB was higher in drought tolerant genotypes, G1 (ILL 6002) and G2 (Indianhead), when compared to the drought sensitive genotypes, G6 (PI 468898) and G7 (ILL 1796) in all the treatments (Figure 3b).
Similar to proline and GB, considerable variation in the total soluble sugar concentration was observed in all the studied genotypes under Si treated and non-Si treated drought stress treatments (Figure 3c). However, the amount of total soluble sugar in only Si treated plants was similar to that of the control treatment.
Figure 3a, b and c. Concentration of proline (μm/g), glycine betaine (GB- μm/g) and total soluble sugars concentration (mg/g) in seven lentil genotypes under different drought stress treatments. ILL 6002 (G1), Indianhead (G2), PBA Jumbo 2 (G3), PBA Nipper (G4), PBA Flash (G5), PI 468898 (G6), ILL 1796 (G7) represent different lentil genotypes. Control (C), drought stress (D), drought stress + Si (DSi) and Si alone (Si) are the different drought stress treatments. Mean values provided with error bars represent the standard error. Different letters denote statistical differences at p ˂ 0.05 within genotypes.
Effects of Si application on the activities of hydrolytic enzymes
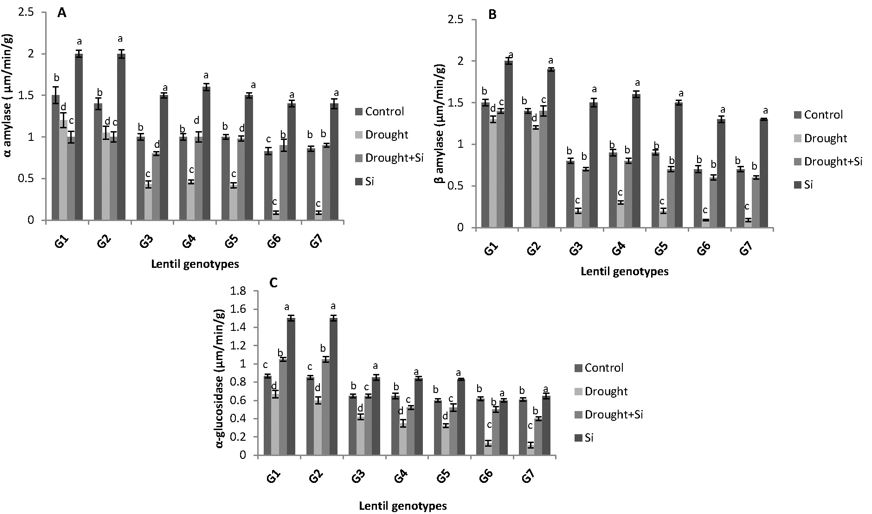
The activity of hydrolytic enzyme (α-amylase, β-amylase and α-glucosidase) decreased significantly in all the genotypes under PEG 6000-induced drought stress and the effect was reversed by the addition of Si (Figures 4a, 4b and 4c). Under control conditions, the drought tolerant genotypes, G1 (ILL 6002) and G2 (Indianhead) showed the maximum α-amylase activity of 1.5 and 1.4μm-1.min-1.g tissue, respectively. The droughtsensitive genotypes G6 (PI 468898) and G7 (ILL 1796), however, displayed lower activity of this hydrolytic enzyme, i.e. 0.83 and 0.86μm-1.min-1.g tissue, respectively. Similar levels of α-amylase activity (1μm-1.min-1.g tissue) were noticed in all the moderately drought tolerant genotypes G3 (PBA Jumbo 2), G4 (Nipper) and G5 (PBA Flash). The percentage of decrease in the α-amylase activity of the genotypes under drought stress was recorded as 30% (G1), 35% (G2), 57% (G3), 54% (G4), 58% (G5), 77% (G6) and 79% (G7).
Si supplementation significantly increased the enzyme activity in all the genotypes under drought stress treatments as seen in Figure 4a. A similar trend was observed for the activity profile of the hydrolytic enzymes, β-amylase and α-glucosidase. β-amylase activity under drought stress ranged from 20% reduction in the drought tolerant genotypes G1 (ILL 6002) and G2 (Indianhead) to 65% in drought sensitive genotypes G6 (PI 468898) and G7 (ILL 1796), when it was compared to the control (Figure 4b). However, the activity of α-glucosidase ranged from 20% in the drought tolerant genotypes G1 (ILL 6002) and G2 (Indianhead) to 50% in drought sensitive genotypes G6 (PI 468898) and G7 (ILL 1796) under drought stress. Si supplied under drought stress significantly enhanced the activity of β-amylase and α-glucosidase in all the genotypes (Figure 4c).
Figure 4a, b and c. Activity of α-amylase, β-amylase and α-glucosidase (μmoles of reducing sugars formed min/g) of the seven lentil genotypes under different drought stress treatments. ILL 6002 (G1), Indianhead (G2), PBA Jumbo 2 (G3), Nipper (G4), PBA Flash (G5), PI 468898 (G6) and ILL 1796 (G7) represent different lentil genotypes. Control (C), drought stress (D), drought stress + Si (DSi) and Si alone (Si) are the different drought stress treatments. Mean values provided with error bars representing the standard error. Different letters denote statistical differences at p ˂ 0.05 within genotypes.
Effects of Si application on the concentration of H2O2, O2− and LPX
Table 3 shows that the concentration of H2O2, O2.− and LPX were significantly higher in response to drought stress in all the lentil genotypes, whereas the concentration in response to DSi treatment showed lower values when compared to the control. The drought tolerant genotypes G1 (ILL 6002) and G2 (Indianhead) showed lower accumulation of ROS and LPX when compared to the drought sensitive genotypes G6 (PI 468898) and G7 (ILL 1796) in all the treatments. An increase of 55% to 70% in H2O2 content was measured in the drought stressed lentil seedlings, whereas a decrease of 60% to 65% was observed in the DSi treatment. Si treatment even lowered the H2O2 content in non-stressed lentil seedlings to 20% to 30 %. Added Si decreased the O2.− content in drought stressed lentil seedlings to 0.5-1-fold, when compared to 1.5-2.5-fold increase under drought stress treatments (Table 3).
MDA is a final product of LPX and its content has been considered as an indicator of oxidative stresses in plants (Mittler, 2002). Significant changes in MDA content in lentil seedlings to drought stress and DSi treatments were observed when compared to their respective controls. Drought stress resulted in a significant increase in LPX in all the lentil genotypes. However, application of Si under drought stress significantly recovered the membrane damage in seedlings as shown from the lower values of LPX. Addition of Si to non-stressed plants did not reveal any significant changes in MDA content (Table 3). Thus, the application of Si seemed to have a protective effect in terms of these parameters under drought stress conditions by lowering the concentration of ROS and LPX.
Table 3. Concentration of H2O2, O2.−, LPX and Si of the seven lentil genotypes under different drought stress treatments. Mean values provided with error bars representing the standard error. Different letters denote statistical differences at p ˂ 0.05 within the genotypes.
Genotypes | Treatments | H2O2 | O2.− (µm-1.ml) | LPX | Si |
|---|---|---|---|---|---|
G1 | Control | 1.22 ± 0.33 | 167.89 ± 0.23 | 0.08 ±0.01 | 0.0042 ±0.006 |
Drought | 2.08 ± 0.01 | 425.36 ± 0.49 | 1.98 ± 0.11 | 0.0132 ± 0.002 | |
Drought+Si | 0.73 ± 0.32 | 153.25 ± 0.52 | 0.97 ± 0.02 | 0.0324 ± 0.021 | |
Si | 0.87 ± 0.01 | 165.28 ± 0.53 | 0.06 ± 0.01 | 0.0067 ± 0.001 | |
G2 | Control | 1.24 ± 0.31 | 163.25 ± 0.27 | 0.07 ± 0.01 | 0.0046 ± 0.003 |
Drought | 2.12 ± 0.01 | 414.25 ± 0.24 | 1.87 ± 0.12 | 0.0142 ± 0.003 | |
Drought+Si | 0.85 ± 0.11 | 162.32 ± 0.78 | 0.77 ± 0.01 | 0.0364 ± 0.022 | |
Si | 0.98 ± 0.21 | 160.25 ± 0.29 | 0.07 ± 0.02 | 0.0051 ± 0.002 | |
G3 | Control | 1.55 ± 0.02 | 215.02 ± 0.25 | 0.15 ± 0.04 | 0.0047 ± 0.011 |
Drought | 3.65 ± 0.14 | 598.21 ± 0.27 | 2.79 ± 0.10 | 0.0125 ± 0.010 | |
Drought+Si | 1.37 ± 0.01 | 204.56 ± 0.45 | 0.85 ± 0.11 | 0.0368 ± 0.011 | |
Si | 1.15 ± 0.01 | 210.89 ± 0.67 | 0.09 ± 0.01 | 0.0072 ± 0.003 | |
G4 | Control | 1.46 ± 0.23 | 211.19 ± 0.59 | 0.15 ± 0.02 | 0.0041 ± 0.023 |
Drought | 3.58 ± 0.11 | 564.32 ± 0.27 | 2.58 ± 0.43 | 0.0361 ± 0.014 | |
Drought+Si | 1.26 ± 0.02 | 201.02 ± 0.46 | 0.72 ± 0.03 | 0.0078 ± 0.004 | |
Si | 1.02 ± 0.87 | 200.07 ± 0.97 | 0.13 ± 0.01 | 0.0067 ± 0.014 | |
G5 | Control | 1.57 ± 0.24 | 208.37 ± 0.83 | 0.14 ± 0.05 | 0.0042 ± 0.011 |
Drought | 3.56 ± 0.52 | 546.32 ± 0.22 | 2.86 ± 0.21 | 0.0328 ± 0.021 | |
Drought+Si | 1.31 ± 0.05 | 200.03 ± 0.25 | 0.65 ± 0.11 | 0.0066 ± 0.013 | |
Si | 1.13 ± 0.11 | 207.90 ± 0.98 | 0.11 ± 0.01 | 0.0049 ± 0.002 | |
G6 | Control | 2.22 ± 0.31 | 255.23 ± 0.21 | 0.22 ± 0.02 | 0.0041 ± 0.023 |
Drought | 3.75 ± 0.02 | 642.35 ± 0.38 | 2.34 ± 0.23 | 0.0375 ± 0.014 | |
Drought+Si | 1.44 ± 0.03 | 224.12 ± 0.27 | 0.67 ± 0.03 | 0.0067 ± 0.020 | |
Si | 1.63 ± 0.11 | 213.25 ± 0.09 | 0.15 ± 0.04 | 0.0071 ± 0.004 | |
G7 | Control | 1.98 ± 0.03 | 257.01 ± 0.27 | 0.26 ± 0.10 | 0.0049 ± 0.021 |
Drought | 3.25 ±0.03 | 655.02 ± 0.36 | 2.45 ± 0.23 | 0.0321 ± 0.011 | |
Drought+Si | 1.21 ± 0.61 | 214.20 ± 0.97 | 0.59 ± 0.11 | 0.008 ± 0.014 | |
Si | 1.54 ± 0.04 | 220.01 ± 0.28 | 0.11 ± 0.01 | 0.0052 ± 0.013 |
Effects of Si application on the activities of antioxidant enzymes
The activity of antioxidant enzymes, APX, POX, CAT and SOD in lentil seedlings increased significantly under drought stress as compared to normal plants. However, Si treatment was found to be effective in enhancing the activity of these enzymes under drought stress and normal conditions (Table 4). The drought tolerant genotypes G1 (ILL 6002) and G2 (Indianhead) showed the maximum activity of all the antioxidant enzymes studied, whereas the drought sensitive genotypes G6 (PI 468898) and G7 (ILL 1796) exhibited the minimum values of enzyme activities in all the treatments.
Table 4. Activity of the antioxidant enzymes APX, POX, (CAT and SOD of the seven lentil genotypes under different drought stress treatments. Mean values provided with error bars represent the standard error. Different letters denote statistical differences at p ˂ 0.05 within genotypes.
Genotypes | Treatments | APX | POX | CAT | SOD |
|---|---|---|---|---|---|
G1 | Control | 0.785 ± 0.012 | 0.321 ± 0.005 | 0.295 ± 0.006 | 0.52 ± 0.06 |
Drought | 1.301 ± 0.014 | 0.687 ± 0.015 | 0.512 ± 0.026 | 1.56 ± 0.01 | |
Drought+Si | 2.010 ± 0.045 | 1.354 ± 0.008 | 0.655 ± 0.014 | 1.87 ± 0.02 | |
Si | 1.110 ± 0.023 | 0.567 ± 0.017 | 0.335 ± 0.062 | 0.88 ± 0.07 | |
G2 | Control | 0.775 ± 0.022 | 0.354 ± 0.012 | 0.275 ± 0.032 | 0.63 ± 0.01 |
Drought | 1.400 ± 0.020 | 0.701 ± 0.012 | 0.495 ±0.008 | 1.67 ± 0.02 | |
Drought+Si | 2.130 ± 0.012 | 1.212 ± 0.007 | 0.665± 0.012 | 1.97 ±0.02 | |
Si | 1.010± 0.011 | 0.435 ± 0.031 | 0.401 ± 0.037 | 0.97 ± 0.01 | |
G3 | Control | 0.301 ± 0.032 | 0.346 ±0.013 | 0.201 ± 0.022 | 0.36 ± 0.05 |
Drought | 0.454 ± 0.045 | 0.762 ± 0.017 | 0.327 ± 0.032 | 0.87 ± 0.02 | |
Drought+Si | 0.735 ± 0.001 | 1.301 ± 0.090 | 0.524 ± 0.014 | 1.27 ± 0.07 | |
Si | 0.387 ± 0.004 | 0.511 ± 0.075 | 0.268 ± 0.016 | 0.67 ± 0.08 | |
G4 | Control | 0.358 ± 0.087 | 0.265 ± 0.036 | 0.213 ± 0.061 | 0.32 ± 0.06 |
Drought | 0.584 ± 0.014 | 0.529 ± 0.033 | 0.361 ± 0.030 | 0.97 ±0.01 | |
Drought+Si | 0.934 ± 0.011 | 1.125 ± 0.028 | 0.556 ±0.018 | 1.89 ± 0.06 | |
Si | 0.398 ± 0.042 | 0.398 ± 0.028 | 0.271 ± 0.008 | 1.1 ± 0.01 | |
G5 | Control | 0.324 ± 0.038 | 0.264 ± 0.003 | 0.221± 0.009 | 0.38 ± 0.03 |
Drought | 0.476 ± 0.009 | 0.545 ± 0.009 | 0.374 ± 0.007 | 0.86 ± 0.02 | |
Drought+Si | 0.791 ± 0.089 | 1.324 ± 0.067 | 0.567 ± 0.013 | 1.76 ± 0.01 | |
Si | 0.443 ± 0.014 | 0.401 ± 0.024 | 0.312 ± 0.010 | 0.67 ± 0.03 | |
G6 | Control | 0.197 ± 0.033 | 0.234 ± 0.025 | 0.175 ± 0.007 | 0.15 ± 0.08 |
Drought | 0.271 ± 0.002 | 0.561 ± 0.008 | 0.241 ± 0.035 | 0.28 ± 0.03 | |
Drought+Si | 0.497 ± 0.045 | 1.526 ± 0.045 | 0.485 ± 0.004 | 1.21 ± 0.04 | |
Si | 0.235 ± 0.014 | 0.339 ±0.023 | 0.265 ± 0.019 | 0.33 ± 0.08 | |
G7 | Control | 0.187 ± 0.012 | 0.231 ± 0.067 | 0.169 ± 0.015 | 0.18 ± 0.07 |
Drought | 0.278 ± 0.007 | 0.615 ± 0.012 | 0.301 ± 0.024 | 0.29 ± 0.09 | |
Drought+Si | 0.505 ± 0.003 | 1.465 ± 0.011 | 0.534 ± 0.023 | 1.35 ± 0.02 | |
Si | 0.261 ± 0.013 | 0.253 ± 0.023 | 0.245 ± 0.011 | 0.76 ± 0.03 |
Although drought stress caused an increase in the activity of APX, it was higher in drought stress supplemented with Si (DSi) treatment than in the other treatments (Table 4). Compared to the control, APX activity was significantly elevated up to 62% to 65% in the drought tolerant genotypes, 50% to 60% in moderately drought tolerant and 35% to 50% in drought susceptible genotypes under drought stress. Si treatment again alleviated the enzyme activity by 52% to 54% in drought tolerant seedlings, 65% to 70% in moderately drought tolerant and 80% to 85% in drought susceptible genotypes when compared to drought stress treatments (Table 4). Compared to the control, POX activity was subsequently increased to 1-2-fold under drought stress treatments in all the studied lentil genotypes. Si application in DSi treatments caused 1-fold, 0.5-1.5-fold and 1-2-fold increase in POX activity of drought tolerant, moderately drought tolerant and drought susceptible genotypes, respectively (Table 4).
CAT activity in drought stressed lentil seedlings increased by 70% to 80% in the drought tolerant genotypes, 60% to 70% in moderately drought tolerant and 30% to 40% in drought susceptible genotypes when compared to the control. However, with Si treatment, CAT activity enhanced by 25% to 35% in drought tolerant genotypes, 50% to 60% in moderately drought tolerant and 75% to 85% in the drought susceptible genotypes. Similar to other antioxidant enzyme activity profiles, SOD activity also increased in drought stressed lentil seedlings, showing a similar trend to other antioxidant enzymes, under both drought stress conditions and DSi treatments. Compared to the control, 1.5-2-fold, 1-2-fold and 0.5-1-fold increase in SOD activity was noticed under drought stress in drought tolerant genotypes, moderately drought tolerant and drought susceptible genotypes, respectively (Table 4). Even though Si application enhanced SOD activity under DSi treatments in all the genotypes, the enhancement was not very significant.
Effect of Si on the concentration of Si in lentil seedlings
In the present study, Si concentration of drought stressed lentil seedlings increased significantly and is probably related to genotypic differences or concentration effect of Si caused by reduced growth due to drought stress (Table 3). Moreover, the exogenous application of Si increased Si concentrations of all the lentil seedlings under drought stress (Table 3).
Multivariate data analysis
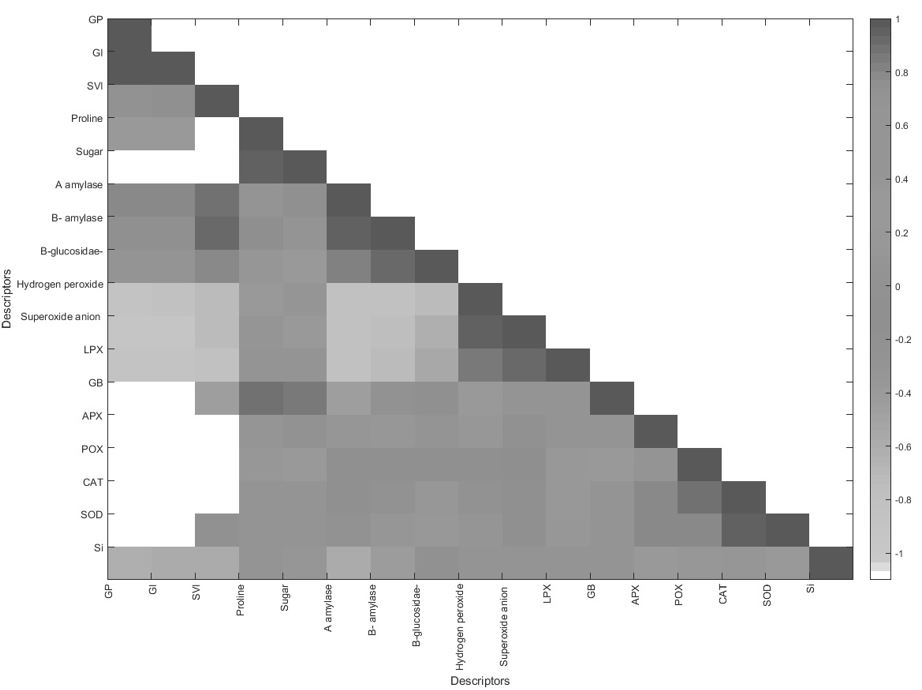
The results from multivariate data analysis from all the four treatments are shown in Figure 5. The PCA obtained from the seven lentil genotypes under control (C), drought stress (D), drought stress supplemented with Si (DSi), and Si alone (Si) including the germination traits (GP, GI and SVI), osmolytes, hydrolytic enzymes, antioxoidant enzymes, H2O2, O2.−, LPX and Si content explained a total of 80.35% (PC1 = 49.7 %; PC2 = 30.60 %) of variability in the data (Figure 5a). The drought tolerance assessment traits (GI, GP, SVI, osmolytes hydrolytic enzymes, antioxidant enzymes and ROS) were significantly correlated among themselves (statistical significance at p ≤ 0.05, Figure 6). Significant positive correlations were observed between GP and GI, SVI and β-amylase, proline and APX, α-amylase and β-amylase, H2O2 and O2.− whereas, GP and LPX, GI and LPX, α-amylase and GB showed significant negative correlations (Figure 6).
From Figure 4a, four distinctive groups can be observed with group 1 corresponding to control treatments separated between tolerant, moderately tolerant and sensitive groups. The same pattern was observed for the rest of the groups. As expected, the drought stress treatment presented the lowest values for germination traits and hydrolytic enzymes (Figures 2a, 2b, 2c, 4a, 4b and 4c) with reduced variation for proline and sugar (Figures 3a and 3b). Group 3 from the DSi treatment exhibited increased drought tolerance trait values for all the genotypes positioning the group in between the drought (group 2) and the control (group 1) treatments. The Si treatment, showed higher levels of the drought tolerance trait values studied for drought tolerance, positioning all the genotypes closer to the control treatment (group 1)
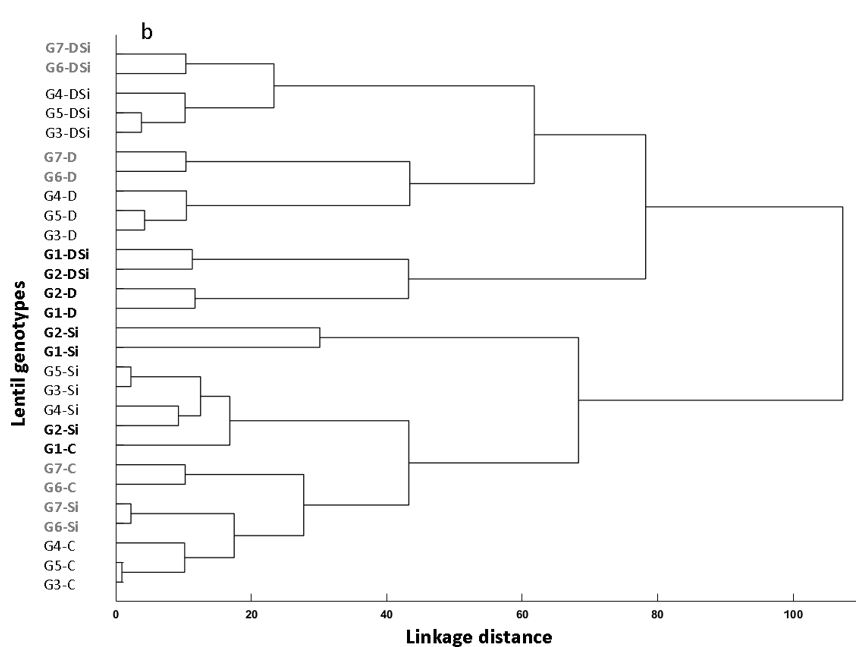
Figure 5a and b. (a) PCA biplot for drought tolerance related traits as vectors and (b) cluster analysis according to the effect of Si on the seven lentil genotypes under control (C), drought stress (D), drought stress + Si (DSi) and Si alone (Si). The abbreviations used in this Figure are ILL 6002 (G1), Indianhead (G2), PBA Jumbo 2 (G3), Nipper (G4), PBA Flash (G5), PI 468898 (G6) and ILL 1796 G7).
Figure 6. Covariance matrix for the drought tolerance related traits studied in seven lentil genotypes.
The cluster analysis clustered the lentil genotypes into different groups based on different drought stress treatments (Figure 5b). The drought tolerant genotypes, G1 (ILL 6002) and G2 (Indianhead) always clustered together under different drought stress treatments. Similar clustering was observed in moderately drought tolerant and drought sensitive genotypes at linkage distance 50.
Discussion
Seed germination traits
Seed germination and seedling emergence are among the most critical and sensitive stages in the lifecycle of plants. Seeds exposed to drought stress may compromise the subsequent seedling establishment and hence the productivity and quality of seeds. Drought is one of the most critical environmental factors limiting lentil productivity in many regions of the world. Some of the studies have shown that lentil plants are sensitive to drought stress during seedling emergence (Muscolo et al. 2014; Mishra et al. 2016).
The decline in water potential gradient between seeds and their surrounding media by the effect of PEG 6000 affects seed germination. A decline in germination traits under drought stress has also been reported in other legumes such as peas (Okcu et al. 2005) and blackgram (Pratap and Sharma, 2010). Blackgram and peas showed a significant decrease in germination percentage, i.e. 70% GP with the osmotic potential of -10 bars and 23% GP with an osmotic potential of -8 bars, respectively.
Results from the present study showed that drought stress negatively affected seed germination, which could be improved by the addition of Si, as it was clearly shown by increase in GP, GI and SVI values, especially for the drought sensitive genotypes G6 (PI 468898) and G7 (ILL 1796) (Figures 2a, 2b and 2c). These results are consistent with studies done in tomatoes (a Si excluder), in which the GI, length and fresh weight of seedlings under PEG-simulated drought stress were significantly improved by Si application (Shi et al. 2014). Previous studies have suggested that Si has a positive effect on the physiology and metabolism of different plants against drought stress (Torabi et al. 2012; Ma and Yamaji, 2008; Liang et al. 2003).
These findings suggested that Si may be involved directly or indirectly in both morphological changes and physiological processes in plants. Results from the present study also showed that during drought stress, Si played a protective role in normal seed germination. This ameliorative effect of Si may be due to its hydrophilic nature by protecting the plants from drought. Irrespective of the drought tolerance capacity, all the lentil genotypes displayed 100% seed germination with Si treatment. Thus, from the results of the current research, Si is shown to be effective in securing 100% seed germination and enhanced drought stress tolerance at the germination and seedling stages.
Fresh and dry weight
When a seed starts to germinate, its fresh weight will increase due to absorption of water. In this study, the seedling fresh and dry weight of all genotypes declined as expected under drought stress conditions, and there were significant differences among the genotypes (Table 2). Fresh weight of drought tolerant genotypes G1 (ILL 6002) and G2 (Indianhead), and drought-sensitive genotypes G6 (PI 468898) and G7 (ILL 1796) decreased under drought stress. The drought tolerant genotypes G1 (ILL 6002) and G2 (Indianhead) had a higher seedling dry weight than the sensitive G6 (PI 468898) and G7 (ILL 1796) under drought stress.
The drought tolerant and moderately drought tolerant genotypes exhibited a reduction in seedling dry weight under drought stress while the drought sensitive genotypes had negligible dry weight showing the sensitivity of these genotypes to drought stress. This could be attributed to reduced photosynthesis, biomass accumulation, respiration and nutrient metabolism (Jaleel et al. 2009). A reduction in plant dry weight under drought stress has also been reported in maize (Ashraf et al. 2007) and chickpeas (Gunes et al. 2007). Si was able to enhance the seedling fresh and dry weight of all the genotypes under non-stress conditions. These results are consistent with Liu et al. (2011) findings, where they reported that Si addition significantly increased the biomass of drought stressed alfalfa seedlings. Thus, this study shows that Si-mediated drought tolerance of lentil seedlings is induced by increased water uptake ability and by modulating sugar levels under stress and non-stress conditions.
Osmolytes (proline, GB and total soluble sugar)
A common stress tolerance mechanism in plants against environmental stresses is the overproduction of different types of osmolytes, such as proline, GB and soluble sugars. The accumulation of these osmolytes in plants might be involved in one or more of the processes such as osmotic adjustment, detoxification of reactive oxygen species, protection of membrane integrity, and the stabilisation of proteins/enzymes and thus contributes to drought tolerance (Blum, 2016). In the present experiment, drought stress resulted in significant accumulation of proline in lentil seedlings. The proline concentration was higher in drought tolerant genotypes, when compared to the drought sensitive and moderately drought tolerant genotypes, as was observed earlier in two drought tolerant lentil genotypes (Muscolo et al. 2014).
Contrary to our findings, Oktem et al. (2008) reported that proline amounts did not differ significantly under drought stress for Turkish lentil genotypes. The present result implies that the accumulation of proline is associated with drought tolerance. Added Si led to a significant decrease in the proline concentration of drought stressed seedlings (Figure 3a), which can be related to the added tolerance effect mediated by Si based on proline biosynthesis/accumulation. The latter can be attributed to the reaction between proline and Si forming silaproline, similar to the mechanism which takes place in humans (Vivet et al. 2000). Similar results were obtained by Gunes et al. (2008) from sunflower genotypes and Mauad et al. (2016) in upland rice plants under drought stress.
GB not only acts as an osmoregulator, but also stabilises the structures and activities of enzymes and proteins, and maintains the integrity of cell membranes against the damaging effects of environmental stresses. PEG-induced drought stress enhanced the concentration of GB in all the lentil genotypes (Figure 3b). Moreover, the drought tolerant genotypes accumulated more GB than sensitive genotypes in all the treatments. Added Si may be responsible for less GB accumulation in all the seedlings which might be a sign of stress injury alleviation. Consequently, regulated levels of GB would make it possible for the plant to regulate low water potential that allows additional water uptake from the stress environment, thus buffering the immediate effect of drought stress within the organism (Blum, 2016).
Drought stress increased the seedling soluble sugar concentration of all lentil genotypes (Figure 3c). This result is consistent with one recent study carried out under drought conditions in lentil genotypes showing an increase in total soluble sugar concentration (Muscolo et al. 2014, Mishra et al. 2016). Compared with the stressed seedlings without additional Si, seedlings with added Si had significantly lower soluble sugar concentration. This shows that drought stress conditions enhanced the anabolism of soluble sugar and adding Si decreased the anabolism of soluble sugar under drought stress. This observation was contradictory to a previous study in which increased soluble sugar levels were observed under Si treatment in drought stressed wheat seedlings (Si accumulator) (Pei et al. 2010). This may be related to the genetic difference between a Si accumulator such as rice, wheat, and sorghum and Si excluder such as lentils and tomatoes.
Further studies using different crops from Si accumulators and Si excluders will ascertain such differences. This result also revealed that under a control and Si treatment without stress conditions, no significant effect of Si on the total soluble sugar level was observed in all the genotypes, which may show that Si application is more effective under stress conditions. Similarly to the case of proline and GB, the highest concentrations of soluble sugar were observed in the drought tolerant genotypes, G1 (ILL 6002) and G2 (Indianhead), when compared to the moderately drought tolerant and drought sensitive genotypes during all treatments. Similar to the present findings, high accumulation of soluble sugar was also noticed in drought tolerant pea and wheat plants when compared to drought sensitive ones (Okcu et al. 2005; Pei et al. 2010).
Hydrolytic enzymes
Starch is the principal storage carbohydrate and its degradation is essential for seed germination. In germinating seeds, starch degradation is initiated by hydrolytic enzymes such as amylases (α and β) and α-glucosidase producing soluble oligosaccharides. These are further hydrolysed by α-amylase to liberate maltose, which is finally broken down by α-glucosidase into glucose, providing energy to the germinating seeds. Conversely, the activity of hydrolytic enzymes in germinating seeds is reduced by drought stress. In the present study, PEG 6000 application resulted in reduced water content in the cells of germinating seeds and as a result, the activity of the hydrolytic enzymes also decreased (Figures 4a, 4b and 4c). However, applied Si resulted in an increased enzyme (β-amylase and α-glucosidase) activity in all the genotypes.
The effect was more pronounced in drought sensitive genotypes, G6 (PI 468898) and G7 (ILL 1796) with an almost 1.5-fold increase in enzyme activity. The seedlings of the drought tolerant G1 (ILL 6002) and G2 (Indianhead), and moderately drought tolerant genotypes PBA Jumbo 2 (G3), Nipper (G4) and PBA Flash (G5) maintained high levels of enzyme activity compared to sensitive genotypes under all the treatments. The increased enzymatic activities of seedlings grown under Si might be explained due to the improved water status of the genotypes with added Si. The drought tolerant genotypes G1 (ILL 6002) and G2 (Indianhead) showed a decline in the activity of α-amylase enzyme with added Si under drought stress when compared to other treatments. However, in the moderately drought tolerant and drought sensitive genotypes, added Si resulted in an increase in α-amylase activity under drought stress.
These results suggest that the effect of Si on the enzyme activity involved in carbohydrate metabolism is quite complex and species-dependent. However, further studies are needed to explore how Si stimulates the desired activity of hydrolytic enzymes under drought stress.
Antioxidant enzymes and reactive oxygen species (ROS)
Many metabolic processes in plant result in the production of reactive oxygen species (ROS), such as superoxide anion (O2.−), hydrogen peroxide (H2O2), and the hydroxyl radical (-OH). Environmental stresses also increase the formation of ROS that oxidises membrane lipids, photosynthetic pigments, proteins and nucleic acids. The increase in LPX has also been reported in many plants under various environmental stresses (Gunes et al. 2007).
Plants with high levels of antioxidants, either constitutive or induced, have been reported to have greater resistance to this oxidative damage. Meanwhile, plants possess efficient antioxidant defence systems for scavenging ROS. APX, POX, CAT and SOD are the major antioxidant enzymes. SOD dismutates O2.− to H2O2 in the chloroplast, mitochondrion, cytoplasm and peroxisome, thus preventing the cellular damage under stress conditions like drought. APX is a component of the ascorbate-glutathione pathway, which plays a key role in scavenging H2O2. POX also plays an essential role in scavenging H2O2, which is a major by-product produced by SOD. CAT eliminates H2O2 by breaking it down directly to form water and molecular oxygen, thus this enzyme does not require a reducing power and has a high reaction rate but a low affinity for H2O2, thereby only removing the high concentration of H2O2.
A significant increase in the O2.− coupled with H2O2 production and LPX observed under PEG-induced drought stress in lentil seedlings clearly indicates an oxidative burst, facilitating cellular damage (Table 3). The increase of O2.− and H2O2 content caused by drought stress followed by increase of LPX in lentil seedlings appears to be alleviated by Si treatments which reduced O2.−, H2O2 accumulation and LPX (Table 3). This might be due to the activity of the compatible solutes such as proline and G, which detoxify ROS by forming a stable complex with them and consequently inhibit LPX (Gunes et al. 2007). These results similar to Si application decreased H2O2 and LPX in leaves of another legume, chickpeas (Gunes et al. 2007), sunflowers (Gunes et al. 2008) and cereal crops such as wheat (Pei et al. 2010) under drought stress. Recently, it is reported that Si application decreased LPX, O2.−, H2O2 accumulation and LPX in liquorice seedlings under drought stress (Zhang et al. 2015).
This study confirmed that Si addition significantly increased the activity of all antioxidant enzymes and resulted in more effective H2O2 dismutation capacity and O2.− elimination power under DSi and Si treatments (Table 4). Higher activities of antioxidant enzymes in DSi than drought stress treatment might play a role in maintaining low levels of H2O2 in the cells under drought stress. Thus, these results suggest that improved activities of antioxidant enzymes induced by addition of Si might protect plant tissues from membrane oxidative damage under drought stress which could significantly contribute to the improvement of drought tolerance. These results are in agreement with the results of Pei et al. (2010), who found that under drought stress, the addition of Si increased the antioxidant activity in wheat plants. This result speculates that Si might be involved in enhancing the expression of genes related to production and activation of antioxidant enzymes in response to drought stress.
Si content
In the current investigation, Si content in lentil seedling was increased by drought stress (Table 3). However, there was no significant variation in Si content among the genotypes. Application of Si under drought stress significantly enhanced Si content in all the genotypes. Although added Si resulted in increased Si content in seedlings under drought stress, lentils are still regarded as a low accumulator of Si, i.e. less than 5mg g dry weight−1 (Meena et al. 2014). The higher content of Si under drought stress in lentils under DSi treatment might be due to deposition of Si in cell walls which can reduce the impacts of drought stress. The deposited Si could strengthen the membranes of plant cells and change their permeability, resulting in improved drought tolerance.
PCA and cluster analysis
The PCA results in this study are in agreement with the criteria established by Sneath and Sokal (1973), who showed that data should represent at least 70% of the total data variability (Figure 5a). The positive correlations were observed among the drought tolerance assessment traits, such as GI, GP, SVI, α-amylase, β-amylase, proline, GB, soluble sugars, antioxidant enzymes and ROS, where the main separator of the treatments was imposed (Figure 6). The latter showed that these traits can be used to assess drought tolerance in plants.
In the PCA, the biplot characterised the genotypes into four distinct groups (1 to 4). Under control (C) condition (group 1), all the genotypes were present near to the origin in the positive direction of the vectors, showing normal behaviour of drought tolerance from the specific genotypes as expected. Whereas, under drought stress (D) treatment (group 2), all the genotypes, especially, the genotypes G6 (PI 468898) and G7 (ILL 1796) moved away from the origin in the negative direction showing less drought tolerance according to germination traits and hydrolytic enzyme activity.
With Si supplementation under drought stress (DSi), the genotypes moved closer towards the origin (group 3), showing improvement in drought tolerance. Interestingly, when Si was given alone without drought stress (Si), all the genotypes were sited away from the origin in the positive direction showing the positive effect of Si in improving the drought tolerance of lentil genotypes. Thus, this study clearly shows the positive role of Si in mitigating drought stress in lentil genotypes and furthermore, the results also showed that Si application can be used to increase seed germination and seedling vigour under drought stress and non-drought stress conditions by improving the response of moderately tolerant and sensitive genotypes. The distinct group formation was also observed through the cluster analysis, which categorised the genotypes into different clusters at a linkage distance 50 based on their drought tolerance levels and the type of drought stress treatment (Figure 5b).
From the results showed here, Si application positively affected drought related parameters and consequently improved the seed germination of lentil genotypes under drought stress. Si ameliorated the effects of drought stress in lentil genotypes by induced water uptake ability, modulating levels of osmolytes, regulating the activities of hydrolytic enzymes and antioxidant machinery. This beneficial effect of Si might be linked to its hydrophilic nature and Si deposited in cell walls allows the plants to keep water, dilute salts and protect tissues from physiological drought. Again, it is worth noting that Si modulates plant metabolism and alters physiological activities, especially in plants under stress than normal environmental conditions.
Si effect was found to be more pronounced for the drought susceptible lentil genotypes compared to moderately drought tolerant and drought tolerant ones. This variation in stress sensitivity of the contrasting lentil genotypes might be linked to a genetic difference in response of genotypes towards drought stress with added Si or it might be due to the significant role of Si in upgrading the water status of the susceptible genotypes more when compared to the moderately drought tolerant and drought tolerant genotypes. However, in-depth investigation is needed to understand how Si regulates plant tolerance to drought stress at the seed germination stage and also the interactions between Si application and plant responses which may help us to better understand the physiological and biochemical functions of Si. In future, the studied lentil genotypes also need to be tested in the field condition to confirm the role of Si in drought tolerance as terminal moisture stress in arid and semi-arid regions is a serious threat that leads to early maturity and low yields of lentil plants.
Conclusion
Drought stress adversely affected seed germination and early seedling growth in all lentil genotypes. The addition of Si for lentil seed germination has been shown to be a beneficial strategy to effectively mitigate the adverse effects of drought stress. However, further studies are required to understand the physiological and biochemical mechanisms of Si-mediated drought stress in higher plants. This study also showed that G1 (ILL 6002) and G2 (Indianhead) are potential drought tolerant lentil genotypes, along with the latest commercial genotype G3 (PBA Jumbo 2) being moderately drought tolerant.
These genotypes could be potentially used as genetic resources for drought tolerance in lentil breeding programs. Furthermore, this is the first report demonstrating the significant role Si plays to alleviate drought stress during seed germination and seedling growth in lentils, especially for moderately drought tolerant and drought sensitive genotypes.
There is still a need to better understand Si functions in more species under different environmental conditions to validate the Si-mediated alleviation of drought stress on a large scale. Again, more detailed studies are needed to explore the physiological, biochemical and molecular mechanisms of Si-mediated drought stress tolerance in plants. Taken together, well-designed, large-scale, and long-term field trials are required to evaluate the feasibility of Si application under drought stress in plants. These results are important and should be part of long-term programs involving Si to boost lentil productivity under drought stress conditions in arid and semi-arid regions.
References
Ahmed, M., Qadeer, U., Ahmed, Z. I., Hassan, F. U., (2016). Improvement of wheat (Triticum aestivum) drought tolerance by seed priming with silicon. Arch. Agron. Soil Sci. 62, 299-315.
Ashraf, M., Foolad, M., 2007. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59, 206-216.
Bates, L., Waldren, R., Teare, I., 1973. Rapid determination of free proline for water-stress studies. Plant and Soil. 39, 205-207.
Bellincampi, D., Dipierro, N., Salvi, G., Cervone, F., De Lorenzo, G., 2000. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. J. Plant Physiol. 122, 1379-1386.
Bergmeyer, H., Grassl, M., Walter, H., 1983. Reagents for enzymatic analysis: Enzymes. Methods of Enzymatic Analysis. 2, 185-223.
Blum, A., 2016. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 40, 4-10. doi: 10.1111/pce.12800.
Chance, B., Maehly, A., 1955. Assay of catalases and peroxidases. Methods enzymol. 2, 764-775.
Chen, G. X., Asada, K., 1989. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 30, 987-998.
Chen, W., Yao, X., Cai, K., Chen, J., 2011. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol Trace Elem Res. 142, 67-76. doi: 10.1007/s12011-010-8742-x.
Coskun, D., Britto, D. T., Huynh, W. Q., Kronzucker, H. J., 2016. The role of silicon in higher plants under salinity and drought stress. Front. Plant. Sci, 7. .
Das, K., Roychoudhury, A., 2016. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Redox Homeostasis Managers in Plants under Environmental Stresses, 53.
Dhindsa, R. S., Plumb-Dhindsa, P., Thorpe, T. A., 1981. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32, 93-101.
Doke, N., 1983. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol. Plant Pathol. 23, 345-357.
Dubois, M., Gilles, K., Hamilton, J., Rebers, P., Smith, F., 1951. A colorimetric method for the determination of sugars. Nature. 168, 167-167. doi: 10.1038/168167a0.
Elliott, C., Snyder, G. H., 1991. Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J. Agric. Food Chem. 39, 1118-1119.
Goliber, T. E., 1989. Gravitational stress and lignification in aerial vs submerged shoots of Hippuris vulgaris. Physiol Plantarum. 75, 355-361.
Grieve, C., Grattan, S., 1983. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant and Soil, 70, 303-307.
Gunes, A., Pilbeam, D. J., Inal, A., Bagci, E. G., Coban, S., 2007. Influence of silicon on antioxidant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress. J. Plant. Interact. 2, 105-113.
Gunes, A., Pilbeam, D. J., Inal, A., Coban, S., 2008. Influence of silicon on sunflower cultivars under drought stress, I: Growth, antioxidant mechanisms, and lipid peroxidation. Commun. Soil Sci. Plant Anal. 39, 1885-1903. .
Hameed A., Sheikh M. A., Jamil A., Basra S. M. A. (2013). Seed priming with sodium silicate enhances seed germination and seedling growth in wheat (Triticum aestivum L.) under water deficit stress induced by polyethylene glycol. Pak. J. Life Soc. Sci. 11 19–24.
Hameed, A., Sheikh, M. A., Jamil, A., Basra, S. M. A., 2013. Seed priming with sodium silicate enhances seed germination and seedling growth in wheat (Triticum aestivum L.) under water deficit stress induced by polyethylene glycol. Pak. J. Life Soc. Sci. 11, 19-24.
Hattori, T., Sonobe, K., Inanaga, S., An, P., Tsuji, W., Araki, H., Eneji, A. E., Morita, S., 2007. Short term stomatal responses to light intensity changes and osmotic stress in sorghum seedlings raised with and without silicon. Environ. Exper Bot. 60, 177-182.
Heath, R. L., Packer, L., 1968. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189-198.
Idrissi, O., Udupa, S. M., De Keyser, E., McGee, R. J., Coyne, C. J., Saha, G. C., Muehlbauer, F. J., Van Damme, P., De Riek, J., 2016. Identification of quantitative trait loci controlling root and shoot traits associated with drought-tolerance in a lentil (Lens culinaris Medik.) recombinant inbred line population. Front. Plant Sci. 7, 1174. doi.org/10.3389/fpls.2016.01174.
Kumar, J., Gupta, S., Gupta, P., Dubey, S., Tomar, R. S. S., Kumar, S., 2016. Breeding strategies to improve lentil for diverse agro-ecological environments. Indian. J. Genet. Pl. Br. 76, 530-549.
Kurdali, F., Al-Chammaa, M., 2013. Growth and nitrogen fixation in silicon and/or potassium fed chickpeas grown under drought and well watered conditions. J. Stress. Physiol. Biochem. 9.
Liang, Y.C., Chen, Q., Liu, Q., Zhang, W., Ding, R., 2003. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 160, 1157-1164.
Liu, H. X., Shen, X. R., Guo, Z.G., 2011. Effects of silicon addition on seed germination and seedling growth of alfalfa [J]. Acta Prataculturae Sinica, 1, 23.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J., 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265-275.
Ma, C. C., Li, Q. F., Gao, Y. B., Xin, T. R., 2004. Effects of silicon application on drought resistance of cucumber plants. J. Soil Sci. Plant Nutr. 50, 623-632.
Ma, J. F., Yamaji, N., 2008. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 65, 3049-3057.
Mauad, M., Crusciol, C. A. C., Nascente, A. S., Grassi Filho, H., Lima, G. P. P., 2016. Effects of silicon and drought stress on biochemical characteristics of leaves of upland rice cultivars. Revista Ciencia Agronomica. 47, 532-539.
McCord, J. M., 2000. The evolution of free radicals and oxidative stress. Am. J. Med. 108, 652-659.
Meena, V., Dotaniya, M., Coumar, V., Rajendiran, S., Kundu, S., Rao, A. S., 2014. A case for silicon fertilization to improve crop yields in tropical soils. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 84, 505-518. doi: 10.1007/s40011-013-0270-y.
Mishra, B., Srivastava, J., Lal, J., Sheshshayee, M., 2016. Physiological and biochemical adaptations in lentil genotypes under drought stress. Russ. J. Plant Physiol. 63, 695-708. doi:10.1134/S1021443716040117.
Muscolo, A., Sidari, M., Anastasi, U., Santonoceto, C., Maggio, A., 2014. Effect of PEG-induced drought stress on seed germination of four lentil genotypes. J. Plant Interact. 9, 354-363.
Noctor, G., Mhamdi, A., Foyer, C. H., 2014. The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol. 164, 1636-1648.
Okcu, G., Kaya, M. D., Atak, M., 2005. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk J Agric For. 29, 237-242.
Oktem, H. A., Eyidooan, F., Demirba, D., Bayrac, A. T., Oz, M. T., Ozgur, E., Selcuk, F., Yucel, M., 2008. Antioxidant responses of lentil to cold and drought stress. J. Plant Biochem. Biotechnol. 17, 15-21. doi:10.1007/BF03263254.
Pei, Z., Ming, D., Liu, D., Wan, G., Geng, X., Gong, H., Zhou, W., 2010. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 29, 106-115. doi:10.1007/s00344-009-9120-9.
Pratap, V., Sharma, Y. K., 2010. Impact of osmotic stress on seed germination and seedling growth in black gram (Phaseolus mungo). J. Env. Biol. 31, 721-726.
Sarker, A., Erskine, W., Singh, M., 2005. Variation in shoot and root characteristics and their association with drought-tolerance in lentil landraces. Genet. Resour. Crop Ev. 52, 89-97. doi:10.1007/s10722-005-0289-x.
Sayed, S., Gadallah, M., 2014. Effects of silicon on Zea mays plants exposed to water and oxygen deficiency. Russ. J. Plant. Physiol. 61, 460-466.
Shen, X., Zhou, Y., Duan, L., Li, Z., Eneji, A. E., Li, J., 2010. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant. Physiol. 167, 1248-1252.
Shi, Y., Zhang, Y., Han, W., Feng, R., Hu, Y., Guo, J., Gong, H., 2016. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant. Sci, 7.
Shi, Y., Zhang, Y., Yao, H., Wu, J., Sun, H., Gong, H., 2014. Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. J. Plant Physiol Biochem. 78, 27-36. .
Shrestha, R., Turner, N., Siddique, K., Turner, D., Speijers, J., 2006. A water deficit during pod development in lentils reduces flower and pod numbers but not seed size. Crop Pasture Sci. 57, 427-438. doi: 10.1071/AR05225.
Siddique, K.H.M., Loss, S.P., Regan, K.L., Jettner, R.L., 1999. Adaptation and seed yield of cool season grain legumes in Mediterranean environments of south-western Australia. Aust. J. Agric. Res. 50: 375-387. doi: org/10.1071/A98096.
Singh, M., Kumar, J., Singh, S., Singh, V. P., Prasad, S. M., 2015. Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev. Environ. Sci. Bio. 14, 407-426.
Sneath, P. H., Sokal, R. R., 1973. Numerical taxonomy. The principles and practice of numerical classification, USA: W. H. Freeman and Company, San Francisco.
Torabi, F., Majd, A., Enteshari, S., 2012. Effect of exogenous silicon on germination and seedling establishment in Borago officinalis L. J. Med. Plants Res. 6, 1896-1901.
Vivet, B., Cavelier, F., Martinez, J., 2000. Synthesis of silaproline, a new proline surrogate. Eur. J. Org. Chem. 807. doi: 10.1002/(SICI)1099-0690(200003)2000:5<807::AID-EJOC807>3.0.CO;2-E.
Yadav, S. S., McNeil, D., Stevenson, P. C., 2007. Lentil-An Ancient Crop for Modern Times. Springer publications.
Yan, M., 2016. Hydro-priming increases seed germination and early seedling growth in two cultivars of Napa cabbage (Brassica rapa subsp. pekinensis) grown under salt stress. J. Hortic. Sci. Biotechnol. 91, 421-426.
Zhang, X., Zhou, D., Cui, J., Ma, H., Lang, D., Wu, X., Wang, Z., Qiu, H., Li, M. 2015. Effect of silicon on seed germination and the physiological characteristics of Glycyrrhiza uralensis under different levels of salinity. J. Hortic. Sci. Biotechnol. 90, 439-443.
Acknowledgements
The research undertaken as part of this project is made possible by the significant financial support in the form of Grain Industry Research Scholarship (GRS) from GRDC - the author would like to thank them for their continued support. The authors are also grateful to University of Melbourne for the Australian Government Research Training Program Scholarship given to Sajitha Biju.
Contact details
Sajitha Biju
School of Agriculture and Food, Faculty of Veterinary and Agricultural Sciences
The University of Melbourne, Parkville, VIC
sbiju@student.unimelb.edu.au
0415 697 330
GRDC Project Code: GRS-11011,
Was this page helpful?
YOUR FEEDBACK