Biology and control options for wild radish, prickly lettuce and sow thistle
Author: Hanwen Wu and Adam Shephard and Michael Hopwood (Wagga Wagga Agricultural Institute, NSW Department of Primary Industries) | Date: 19 Feb 2019
Take home messages
- Long persistence of wild radish requires management programs of more than six years.
- Wild radish emergence occurred mainly in the second season after fresh seed release from the parent plant, whereas sowthistle and prickly lettuce had their major emergence in the first season.
- Don’t let in-crop wild radish patches go to seed.
- Seeds of sowthistle and prickly lettuce are relatively short-lived, therefore prevention of seed set over two years should be effective in running down the seedbank.
- Seeds of sowthistle and prickly lettuce are surface germinators, but prickly lettuce can also emerge beyond 5cm burial depth.
- Good control within paddocks is not the full solution. It is also important to control weeds on fenceline, roadsides and other non-cropping areas, particularly for sowthistle and prickly lettuce due to their wind-dispersal capabilities.
- Seedbank management is critical for the three weeds. It requires a combination of chemical and non-chemical options as there is no single ‘silver bullet’ available.
Background
Weeds are persistent problems in agriculture, increasing production costs and reducing crop yields. The total cost of weeds to Australian grain growers is estimated at $3,318 million, including $2,573 million for expenditure on control options and $745 million for revenue loss (Llewellyn, 2016). Wild radish (Raphanus raphanistrum L.) and common sowthistle (Sonchus oleraceus L.) are among the top 10 worst weeds nationally and prickly lettuce (Lactuca serriola L.) was ranked number 18 in the southern region (Llewellyn, 2016).
Both sowthistle and prickly lettuce belong to the sunflower family (Asteraceae). Seeds of sowthistle and prickly lettuce are enclosed singly in small hard achenes equipped with a pappus, indicating that the spread of the two wind-blown weeds across agricultural landscapes could be very rapid through long distance dissemination via surface runoff and water movement in irrigation channels and waterways.
Control of these three broadleaved weeds relies heavily on herbicides, which has resulted in the rapid development of herbicide resistance to Groups B, C, F, I and M in wild radish, and to Groups B and M in sowthistle and prickly lettuce. Diversifying control options by incorporating chemical and non-chemical measures is the only way forward if resistant weeds are to be effectively managed. An understanding of weed biology would assist in identifying the weakest link in the weed life cycle and hence contribute to the design of effective control programs. New knowledge on weed biology and management is presented in this paper, with particular focus on prickly lettuce due to the limited information available.
Wild radish biology and management
Wild radish is of Mediterranean origin and is a major weed of winter crops in southern Australia (Murphy et al. 1999). The major source of spread is due to contamination in grain, chaff and hay. It is highly competitive in crops and can cause a yield loss of 10%-90% in cereals, canola and pulses (Storrie, 2014). It germinates in a wide range of temperatures from 5°C to over 35°C, with optimal diurnal fluctuation of 25°C/10°C. Wild radish can therefore emerge at any time of the year given sufficient soil moisture. However, wild radish predominantly emerges in late autumn (May) and early winter, followed by staggered emergence throughout the season (Young, 2001). Deeper burial depths often result in decreased emergence but at the expense of increased persistence. Strong dormancy and long persistence are the two most important features contributing to the difficulty in control. Fresh seeds of wild radish are dormant and many seeds will not germinate until the second season after their formation (about 18 months later) (Storrie, 2014). Wild radish seed has a longevity of more than six years. Two critical steps are required to effectively manage wild radish: (1) prevent seed return to the seedbank (seedset control) and; (2) deplete the existing seedbank (through emergence and seed decay).
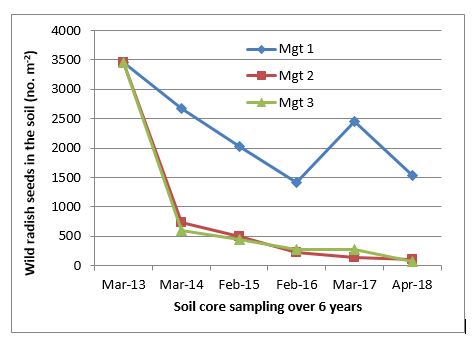
Without the new addition of fresh seeds, control measures have to be in place for more than six years in order to exhaust the seedbank (Cheam, 1996), which is supported by results from a long-term rotation trial established in Wagga Wagga, i.e. 2013 field peas → 2014 barley → 2015 triazine tolerant (TT) canola → 2016 wheat → 2017 barley → 2018 wheat.
Over the six year period, the management (Mgt) 1 (treatment pre-emergent (PRE)) had much higher wild radish seeds in the seedbank than Mgt 2 (treatment PRE + post-emergent (POST)) and Mgt 3 (treatment PRE + POST + Seedset) (Figure 1). Mgt 2 and 3 were equally effective, resulting in a rapid decline of wild radish seedbank in the first year, followed by a steady decline over the remaining years. The results indicate that the wild radish seeds persist well in the soil, requiring a long-term approach of more than six years to manage the wild radish problem. Any new seed replenishment will waste the previous management efforts.
Figure 1. Seedbank dynamics of wild radish between 2013 and 2018 under three management options. Mgt 1– Pre-emergent treatments only (PRE), Mgt 2 – PRE followed by post-emergent treatments (PRE + POST), and Mgt 3 – (PRE + POST) followed by late seedset control (Seedset).
Herbicides are a valuable tool for wild radish control. For effective control, it is necessary to target young and actively-growing weeds with a rosette of less than 5cm in diameter. A two-spray herbicide strategy is recommended for high levels of in-crop control (Storrie, 2014). The ‘two-spray’ strategy on wild radish was also evaluated in two field trials in southern NSW (Wagga Wagga and Marrar, 2015).
The field had a natural infestation of wild radish at 110 plants/m2 and 78 plants/m2 at Wagga Wagga and Marrar sites, respectively. A pre-sowing double-knockdown with glyphosate followed by paraquat was used to control the emerged wild radish. Wheat (cv. Corack) was sowed at Wagga Wagga site on 1 June 15 and oats (a mixture of cv. Yiddah and Mitika) sowed at Marrar site on 9 May 2015.
The two-spray strategies were highly effective on wild radish control. The 1st spray of Bromicide® MA followed 40 days later by each of the fourteen 2nd spray treatments had 100% control of wild radish (Table 1). However, Bromicide® MA without a follow-up spray only achieved 85% control.
Without the first spray of Bromicide® MA, five 2nd spray treatments Amicide® 700, Jaguar®, Broadside®, Bromicide® MA and a mixture of ParadigmTM + LVE Polo 570 (LVE MCPA) gave poor control of wild radish (<90%). Bromicide® MA applied on 16 September had only 30% control of wild radish as compared to the same treatment applied on 7 August (85%).
Table 1. Two-spray strategy on wild radish control in wheat (Wagga Wagga 2015).
First spray treatments | ||||||
|---|---|---|---|---|---|---|
Second spray treatments | Visual rating (%) | Plant density (plant/m2) | ||||
Treatment | Rate (ml or g/ha) | Rate (g a.i./ha) | Untreated | Bromicide MA | Untreated | Bromicide MA |
Amicide 700 + Liase | 1400 ml +2 % | 980 | 87 | 100 | 0.3 | 0 |
Precept + Hasten | 2000 ml + 1% | (250 + 50) | 100 | 100 | 0.0 | 0 |
Velocity + Hasten | 1000 ml + 1% | (210 + 37.5) | 97 | 100 | 3.3 | 0 |
Tigrex | 1000 ml | (250 + 25) | 97 | 100 | 0.2 | 0 |
Jaguar | 1000 ml | (250 + 25) | 17 | 100 | 5.5 | 0 |
Broadside | 1000 ml | (280 + 140 + 40) | 80 | 100 | 0.8 | 0 |
Flight EC | 720 ml | (25.2 + 151.2 + 252) | 93 | 100 | 0.0 | 0 |
Paragon | 500 ml | (250 + 25) | 98 | 100 | 0.0 | 0 |
Bromicide MA | 1400 ml | (280 + 280) | 30 | 100 | 0.8 | 0 |
Affinity Force + Agritone 750 | 100 ml + 330 ml | 24 + 250 | 97 | 100 | 1.2 | 0 |
Paradigm + LVE Polo 570 + Uptake | 25 g + 440 ml + 0.5% | (5 + 5) + 250 | 87 | 100 | 1.3 | 0 |
Logran 750 WG + Agritone 750 + Uptake | 15 g + 330 ml + 1% | 11.25 + 250 | 100 | 100 | 0.0 | 0 |
Triathlon | 1000 ml | (250 + 150 + 25) | 99 | 100 | 0.3 | 0 |
Untreated control | 0 | 85 | 16.7 | 0 | ||
Lsd0.05 | 8.17 | 4.35 | ||||
Note:1st spray at 4-5 leaf stage on 7 August using Bromicide MA (200 g/L bromoxynil + 200 g/L MCPA) applied at 1400 ml/ha. 2nd spray treatments on 16 September 40 DAT1 (Days after treatment). Visual rating assessed on 23 October, 37 DAT2 and plant density on 9 November, 54 DAT2.
At Marrar site (Table 2), the 1st spray treatments without the 2nd spray were not effective on wild radish (7%-83%). In the untreated 1st spray treatments, only three single knock 2nd sprays of Precept®, Flight® EC and GalleryTM 750 + LVE Polo 570 achieved excellent control of wild radish (98%-100%), while the control for the remaining six 2nd spray treatments was poor (17%-87%).
The combination of either Jaguar® + Sencor® or Tigrex® + Sencor® as the 1st spray treatment, followed 25 days later by any of the nine 2nd spray treatments achieved high level of control (98%-100%). However, the two-spray program using Igran® + Agritone 750 as the 1st spray treatment, even with the follow-up application of nine 2nd sprays, did not achieve consistent control, with the 2nd spray treatments Amicide® 700 and Affinity® Force + Agritone 750 being poorly effective (80%-83%).
Table 2. Visual control rating (%) of two-spray strategy on wild radish in forage oats at 60 days after the 2nd spray (Marra 2015).
First spray treatments | ||||||
|---|---|---|---|---|---|---|
Second spray treatments | ||||||
Treatment | Rate (ml or g/ha) | Rate (g a.i./ha) | Untreated | Jaguar + Sencor | Tigrex + Sencor | Igran + Agritone 750 |
Amicide 700 + Liase | 1150 ml +2 % | 805 | 40 | 100 | 100 | 83.3 |
Precept + Hasten | 2000 ml + 1% | (250 + 50) | 98.3 | 100 | 100 | 100 |
Velocity + Hasten | 1000 ml + 1% | (210 + 37.5) | 83.3 | 98.3 | 100 | 93.3 |
Tigrex | 1000 ml | (250 + 25) | 80 | 100 | 100 | 96.7 |
Flight EC | 720 ml | (25.2 + 151.2 + 252) | 100 | 100 | 100 | 100 |
Paragon | 500 ml | (250 + 25) | 86.7 | 100 | 100 | 95 |
Affinity Force + Agritone 750 | 100 ml + 330 ml | 24 + 250 | 68.3 | 100 | 100 | 80 |
LVE Polo 570 | 440 ml | 250 | 63.3 | 100 | 100 | 98.3 |
Gallery 750 + LVE Polo 570 + Uptake | 100 g + 440 ml + 0.5% | 75 + 250 | 100 | 100 | 100 | 100 |
Untreated control | 0 | 83.3 | 80 | 6.7 | ||
Lsd0.05 | 12.19 | |||||
Note: 1st spray treatments on wild radish at 5-6 leaf stage on 23 July. 2nd spray treatments on 17 August, 25 DAT1. Visual rating assessed on 16 October, 54 DAT2. Products and formulations applied in the first application include Jaguar® (250 g/L bromoxynil + 25 g/L diflufenican) + Sencor® 480 SC (480 g/L metribuzin ) applied at 1000 ml + 100 ml/ha, Tigrex® (250 g/L MCPA and 25 g/L diflufenican) + Sencor® 480 SC (480 g/L metribuzin ) at 1000 ml + 100 ml/ha; and Igran® 500 SC (500 g/L terbutryn) + Agritone 750 (750 g/L MCPA) at 500mL + 350mL/ha.
Sowthistle biology and management
Common sowthistle originates in Europe. The weed is not known to compete heavily with cereal crops. However, in a poorly competitive crop, common sowthistle contributes to green matter at harvest and can lead to grain quality problems. It can produce up to 25,000 seeds/plant (Widderick, 2017) and 68,000 seeds/m2 in a fallow (Storrie, 2014), indicating seedbank can be built up very quickly. The fresh seeds were previously believed to be dormant. However, our recent research showed that seeds from different locations and seasons exhibited variable dormancy based on 38 populations collected in southern NSW, with dormancy ranging from 5.3% to 98.7%.
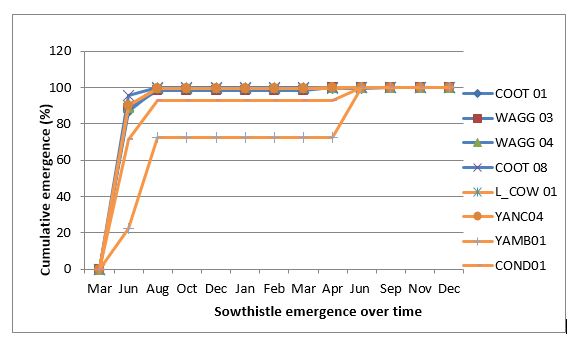
Sowthistle is a surface germinator, with most emergence occurring from seed on or near the soil surface and no emergence from soil depth of more than 5cm (Chauhan et al. 2018). However, deep burial through cultivation could increase seed persistence. Sowthistle seeds can germinate over a broad range of temperatures from 5°C to 35°C, with maximum germination at alternating day/night temperature of 20/12°C. Sowthistle can therefore grow and flower all year round as long as soil moisture is not limiting. However, majority of plants emerged between April and August in southern NSW, with most emergence in the first year after fresh seed burial on soil surface, and little emergence in the second year. Exception was the samples YAMB01 and COND01 which had some emergence in the second year after burial (Figure 2).
Figure 2. Emergence dynamics of sowthistle collected from different rainfall environments (tested in trays under field conditions in Wagga Wagga from March 2016 to December 2017).
Growing competitive cereals is an effective non-chemical option for sowthistle control (Widderick, 2017). Recently research has shown that high plant population and narrow row spacing are also effective in suppressing sowthistle in the traditionally-believed ‘poorly competitive’ faba bean and chickpea crops.
Higher faba beans populations resulted in lower plant height and biomass of sowthistle in both narrow- and wide-row spacing treatments (Table 3). Crop population at 60 plants/m2 reduced the sowthistle seed head/plant, seeds/plant and seeds/m2 as compared to the crop population at 15 plants/m2 and the Nil-crop control.
Narrow-row spacing in the faba bean also reduced sowthistle plant height, plant biomass, seed head/plant and seeds/plant by 13%, 52%, 53% and 54%, respectively, when compared to the wide-row spacing treatment.
Table 3. Faba beans populations and row spacings on sowthistle growth and seed production.
Faba beans target population (plants m-2) | Row spacing (cm) | Plant height cm | Plant biomass (g/plant) | biomass (g/m2) | Seeds/ plant | Seeds/m2 |
|---|---|---|---|---|---|---|
15 | 46 | 70.0 | 9.8 | 46.4 | 12543.9 | 56372.1 |
30 | 46 | 66.9 | 6.7 | 38.6 | 7483.4 | 32675.8 |
60 | 46 | 64.2 | 5.2 | 30.3 | 5253.7 | 21634.9 |
Nil-crop | na | 73.6 | 15.1 | 115.4 | 9341.5 | 51442.6 |
15 | 23 | 56.60 | 4.74 | 54.86 | 5522.7 | 46219.4 |
30 | 23 | 61.19 | 3.49 | 28.61 | 3432.4 | 22575.9 |
60 | 23 | 56.42 | 2.22 | 13.47 | 2639.8 | 13010.8 |
Nil-crop | na | 75.56 | 13.41 | 120.97 | 8348.3 | 78981.6 |
Lsd0.05 | ||||||
Population | 7.73** | 3.89** | 40.90** | 3628.9* | 20523.2** | |
Row spacing | 5.47* | 2.75* | 28.92 | 2566.0** | 14512.1 | |
na: not applicable.
The impact of chickpea population on sowthistle growth and seed production in the chickpea trial was similar to faba beans. Higher chickpea populations had significantly lower sowthistle plant height, biomass, seeds/plant and seeds/m2 at both row spacing treatments (Table 4).
There were no significant differences between the narrow and wide row spacing treatments. .
Table 4. Chickpea populations and row spacings on sowthistle growth and seed production.
Chickpea target population (plants m-2) | Row spacing (cm) | Plant height (cm) | Biomass (g/plant) | Biomass (g/m2) | Seeds/ plant | Seeds/m2 |
|---|---|---|---|---|---|---|
20 | 46 | 72.4 | 13.2 | 132.3 | 5499.2 | 29924.4 |
40 | 46 | 62.7 | 4.1 | 74.4 | 2045.0 | 10914.5 |
80 | 46 | 61.4 | 4.5 | 87.2 | 2267.2 | 10688.7 |
Nil crop | na | 66.0 | 16.7 | 91.8 | 5618.2 | 37442.0 |
20 | 23 | 75.4 | 8.1 | 79.1 | 5652.7 | 22894.6 |
40 | 23 | 66.3 | 3.3 | 31.8 | 4030.9 | 15534.4 |
80 | 23 | 60.6 | 2.7 | 20.1 | 1734.2 | 6658.2 |
Nil crop | na | 85.4 | 16.2 | 265.1 | 10160.4 | 45333.6 |
Lsd0.05 | ||||||
Population | 10.35* | 3.11** | 85.1* | 1545.4** | 10791.6** |
na: not applicable.
Prickly lettuce biology and management
Prickly lettuce is of Eurasian origin and it was first recorded in 1899 in the Upper Hunter region of NSW. It has recently become an increasing problem in cereals and lucerne pastures in southern NSW. Similar to sowthistle, it did not cause significant yield loss in cereals or grain legumes, but grain quality and harvesting efficiency were severely compromised (Amor 1986a). Flowering buds are cut together with grain during harvest, resulting in grain contamination and reductions in value. Plants are difficult to control with herbicides once the plants start to elongate. Mechanical control is ineffective as it regrows with competitive branches after cutting or harvesting and progresses to set seeds (Amor 1986a).
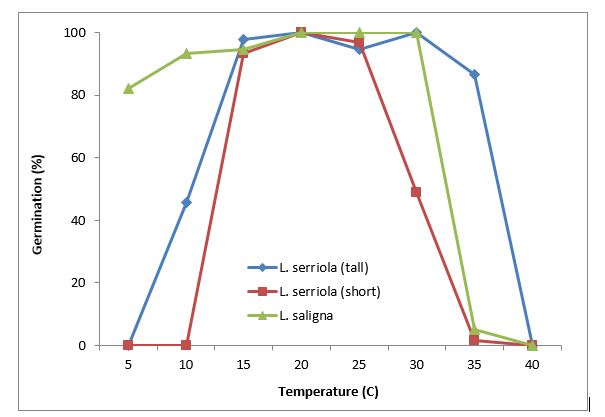
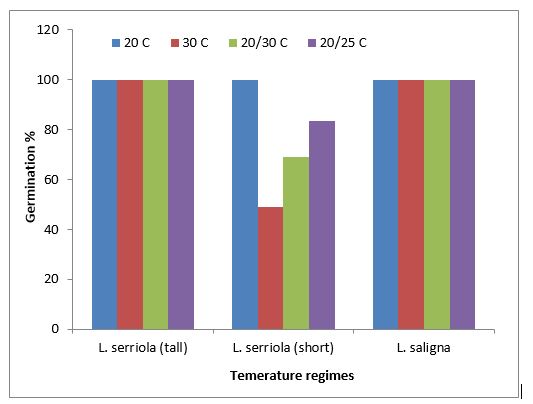
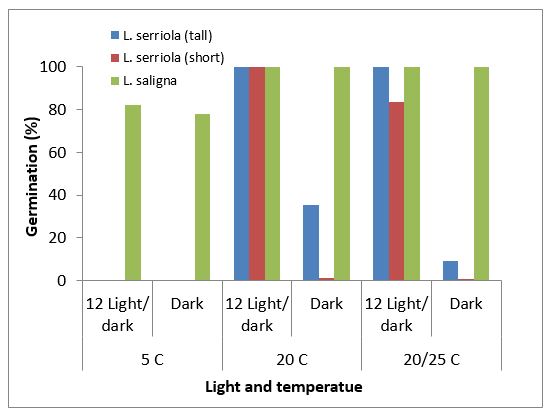
In Australia, Amor (1986b) estimated that prickly lettuce plant in crop stubble produced 48,000 seeds/plant while it produced 900 seeds/plant in a wheat crop. The seed has been associated with its rapid dispersal over distances of up to 43km (Lu et al. 2007). Prickly lettuce has wide germination temperatures, ranging from less than 5°C to more than 35°C, depending on the species and population (Figure 3). Willow-leaf lettuce (Lactuca saligna) had 80% germination even at 5°C. The optimum temperatures for germination were 15 – 25°C for L. serriola. Alternating temperature did not improve the germination of L. serriola (tall) and L. saligna, but reduced the germination of L. serriola (short) (Figure 4).
Figure 3. Germination temperature requirements for prickly lettuce.
Figure 4. Impact of alternating temperatures on the germination of prickly lettuce.
Light stimulated the germination of L. serriola for tall and short biotypes, while light was not required for the germination of L. saligna (Figure 5).
Figure 5. Impact of light on the germination of prickly lettuce.
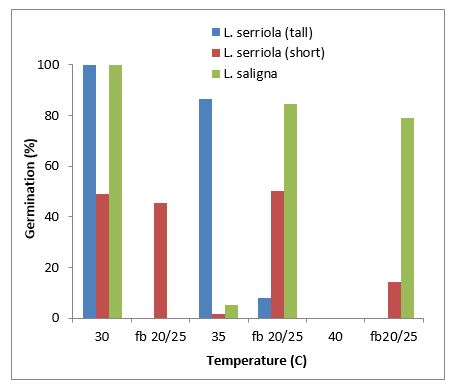
Higher temperatures induced secondary dormancy in prickly lettuce (Figure 6). L. serriola seed (short biotype) underwent induced dormancy at 30 °C, while both biotypes of L. serriola as well as the L. saligna entered secondary dormancy at 35 and 40 °C.
Figure 6. Dormancy of prickly lettuce induced at higher temperatures of 30, 35 and 40 °C.
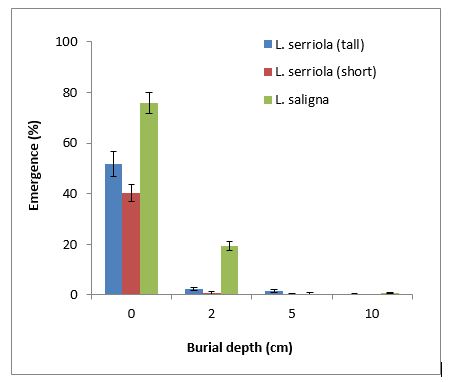
Prickly lettuce had major emergence (40% to 76%) from the soil surface (0cm), depending on the species and population, with L. saligna still having 19% at 2cm burial depth. Both Lactuca species can emerge (0.25% to 0.5%) at deeper depths (5cm and 10cm), which is different to sowthistle, indicating cultivation to bury seeds will be less effective in prickly lettuce than sowthistle.
Figure 7. Impact of burial depth on the emergence of prickly lettuce under glasshouse conditions.
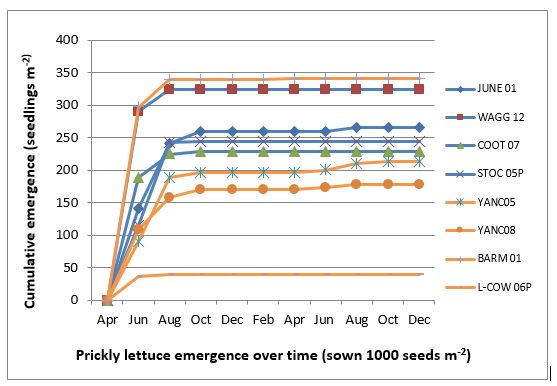
Prickly lettuce populations differed in their final cumulative emergence, ranging from 39 plants/m2 to 341 plants/m2 (Figure 8), however, they had similar emergence patterns, with 69% emergence in late autumn and early winter, 27% in later winter, 2.4% in spring in the first year after burial and only 1.6% emergence between autumn and winter in the second year (Wu et al. 2018).
Figure 8. Emergence of prickly lettuce in seedling trays without crop competition under field conditions in Wagga Wagga from April 2016 to December 2017. The number of seeds sown onto each tray was expressed as seeds/m2.
Effective herbicide options are limited on prickly lettuce. A herbicide trial on prickly lettuce in summer fallows identified that five treatments, glyphosate, 2,4-D amine + glyphosate, metsulfuron-methyl + glyphosate, glufosinate ammonium and fluroxypyr achieved good control with more than 90% mortality, while the remaining 11 treatments only controlled 30%–88% of prickly lettuce. The follow-up ‘double-knock’ treatment with paraquat at 600 g a.i./ha provided 100% control on prickly lettuce, even in the untreated plots which did not have the first knock of herbicide applications (data not shown).
Table 5. Herbicide control efficacy on mature prickly lettuce plants at Lake Cowal.
Treatment | Rate (ml or g ha-1) | Rate (g a.i./ha) | Single_knockA | |
|---|---|---|---|---|
Visual ratingA (%) | Density (plants m-2)B | |||
Amicide Advance 700 + Liase | 1150 ml + 2% | 805 | 30.0 | 3.7 |
Amicide 700 Advance + weedmaster ARGO + LI700 | 515 ml + 1300 ml + 0.3% | 360 + 700 | 93.3 | 0.6 |
Weedmaster ARGO + LI700 | 1300 ml + 0.3% | 700 | 90.0 | 2.0 |
Starane Advanced + Uptake | 600 ml + 0.5% | 200 | 95.0 | 0.8 |
Starane Advanced + Weedmaster ARGO | 600 ml + 1300 ml | 200 + 700 | 88.3 | 2.4 |
Ally + Weedmaster ARGO | 7 g + 1300 ml | 4.2 + 700 | 95.0 | 1.6 |
Goal + Weedmaster ARGO + BS1000 | 75 ml + 1300 ml +1% | 18 + 700 | 86.7 | 2.1 |
Kamba + Weedmaster ARGO | 240 ml + 1300 ml | 120 + 700 | 85.0 | 4.5 |
Basta | 4000 ml | 800 | 91.7 | 1.8 |
Amitrole T + LI700 | 5600 ml + 0.3% | (1400 + 1230) | 85.0 | 1.6 |
Tordon 75D | 700 ml | (210 + 53) | 53.3 | 3.9 |
Tigrex | 1000 ml | (250 + 25) | 81.7 | 3.7 |
Affinity Force + Agritone 750 | 100 ml + 330 ml | (24 + 250) | 76.7 | 2.5 |
Lontrel 300+ LVE Agritone 570 | 150 ml +1000 ml | 45 + 570 | 63.3 | 2.4 |
LVE Agritone 570 | 1000 ml | 570 | 40.0 | 4.3 |
Hotshot + LVE Agritone 570 | 750 ml +1000 ml | (7.5 + 105) + 570 | 86.7 | 3.5 |
Control | 0.0 | 6.2 | ||
Lsd0.05 | 17.93 | 1.89 | ||
ATreatments applied on 10 December 2015 and a visual rating was conducted on 8 January 2016, 29 DAT. BThe surviving prickly lettuce plants were recorded on 2 February 2016, 64 DAT.
References
Amor, R.L. (1986a). Incidence and growth of prickly lettuce (Lactuca serriola L.) in dryland crops in the Victorian Wimmera. Plant Protection Quarterly. 1, 148–151.
Amor, R.L. (1986b). Chemical control of prickly lettuce (Lactuca serriola) in wheat and chick-peas in the Victorian Wimmera. Plant Protection Quarterly. 1, 103–105.
Chauhan, B.S., Widderick, M., Werth, J. and Cook, T. (2018). Northern IWM factsheet - Common Sowthistle (Sonchus oleraceus L.): Ecology and Management. https://weedsmart.org.au/wp-content/uploads/2018/09/Common-Sowthistle-Sonchus-oleraceus-L_-ecology-and-management.pdf.
Cheam, A.H. (1996). Wild radish: relating its biology to its resilience. Proceeding of a one-day wild radish workshop held at Orage in March 1996.
Llewellyn, R.S., Ronning, D, Ouzman, J., Walker, S., Mayfield, A. and Clarke. M. (2016). Impact of weeds on Australian grain production: the cost of weeds to Australian grain growers and the adoption of weed management and tillage practices Report for GRDC. CSIRO, Australia.
Lu, Y.Q., Baker, J. and Preston, C. (2007). The spread of resistance to acetolactate synthase inhibiting herbicides in wind borne, self-pollinated weed species, Lactuca serriola L. Theoretical Applied Genetics. 115, 443-50.
Murphy, C., Lemerle, D., Medd, R. and Cullis, B. (1999). Manipulation of wild radish emergence to accelerate seed bank decline: preliminary finds. Proceedings of 12th Australian Weeds Conference, Hobart, pp 256-260.
Storrie, A. M. (ed). (2014). Integrated weed management in Australian cropping systems. Grains Research and Development Corporation.
Widderick, M. (2017). Sowthistle – know the enemy, exploit its weakness. https://ahri.uwa.edu.au/sowthistle-know-the-enemy-exploit-its-weakness/.
Wu, H.,Shephard, A. and Hopwood, M. (2018). Emergence patterns and herbicide control of prickly lettuce (Lactuca serriola L.). Proceedings of the 21st Australasian Weeds Conference, pp. 290-294. 9 –13 September 2018, Sydney, New South Wales.
Young, K.R. (2001). Germination and emergence of wild radish (Raphanus raphanistrum L.). PhD thesis. The University of Melbourne.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC — the author would like to thank them for their continued support.
Contact details
Hanwen Wu
Wagga Wagga Agricultural Institute
NSW Department of Primary Industries
Pine Gully Road, Wagga Wagga, NSW 2650
02 69381602
hanwen.wu@dpi.nsw.gov.au
GRDC Project Code: : UA00149, UA00156 and US00084,
Was this page helpful?
YOUR FEEDBACK