Factors that drive nitrogen availability in wheat, including soil microbiology
Author: Vadakattu V.S.R. Gupta and Therese McBeath (CSIRO Agriculture and Food, Waite Campus, SA) and Victor Sadras (SARDI, Waite Campus, SA) | Date: 12 Feb 2019
Take home messages
- Microorganisms associated with the soil, crop residues, roots and in the rhizosphere of cereal crops regulate nitrogen (N) cycling processes, mediate its availability to plants and its losses from the system.
- Cereal crops differ in terms of microbial biomass, activity and levels of different N cycling processes within the crop and hence N availability.
- Significant variation exists between wheat varieties in terms of general microbial composition and microbial groups involved in N cycling processes.
- Selection of varieties that are more readily associated with more effective groups of N cycling microbes could be a sustainable option to increase N use efficiency in cereal crops.
- The process of N immobilisation (tie-up) by the microbial biomass (MB) which affects the availability of N from soil organic matter and fertiliser early in the growing season should be considered in N management decisions, especially for cereal crops, in stubble retained and intensive cereal cropping systems.
Background
N fertiliser inputs account for a large proportion of crop production costs and can contribute to the risk of losses in dry seasons. Over the last five decades, varietal selection for yield has steadily increased crop N uptake in wheat (Aziz et al., 2017). Increased N uptake combined with cropping intensification and reduced frequency of pastures results in the mining of Australian soils and compromised protein levels in cereal crops. To maintain yield and protein, the current N fertiliser rate (approximately 45kg N per ha on average) will need to double if the crop’s ability to capture mineral N from soil, which is <50% of applied fertiliser N, is not improved further (Angus and Grace, 2017; Gupta, Kirkegaard et al., 2018, GRDC project CSP00186). Assuming $1 per kg N, and 2.54 million hectares of wheat and barley in South Australia (2015-16 census) and an additional 45kg of fertiliser N per ha input, this represents $114 million annually for South Australian growers alone.
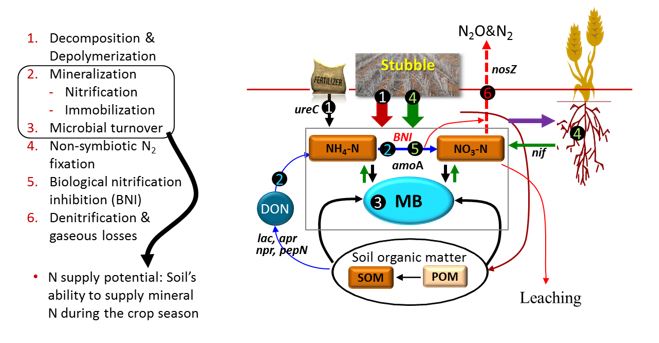
Microorganisms (microbiome) associated with the soil, plant residues, roots and the rhizosphere regulate N cycling and microbial processes, mediate its availability to plants and its losses from the system. A diverse group of microbial communities are involved in the release of nitrate N from soil organic matter (SOM), input through N fixation and N losses, and they are present in all agricultural soils. There are direct links between processes involved in the release of N from SOM and those involved in the transformation of fertiliser N to plant available forms e.g. conversion of urea to nitrate N (ureC, amoA genes) (Figure 1). Therefore, management strategies that manipulate microbial communities controlling N release, mineralisation and immobilisation would help optimise both organic and fertiliser N use efficiency, in particular in cereal crops.
Figure 1. Biological processes involved in N cycling that influence N availability to cereal crops. SOM – soil organic matter, DON – dissolved organic nitrogen, POM – particulate organic matter, MB - microbial biomass. Microbial groups (genes) involved in specific N cycling processes are shown in italics.
Plants are known to shape their rhizosphere microbiome by selectively promoting different members of the soil bacterial and fungal community. The rhizosphere, rhizoplane (root surface) and endosphere (root internal region) contain distinct but reproducible microbial communities. The plant’s microbiome and above- and below-ground associated microbiomes, are an integral part of the wider genome of the plant and are now considered as the extended phenotype of all plants. Recent knowledge based on new molecular techniques suggests that capturing N benefits from improved plant-microbe interactions would be a more sustainable option for improved nutrient and water use efficiency in crops, and overall production and profit. For example, clear differences in bacterial and fungal communities have been observed in the domesticated modern bread wheats, landraces and wild relatives within the Triticeae (Gupta VVSR and Richardson A, CSIRO, unpublished). Therefore, successful exploitation of soil microbial capabilities requires the development of the plant-microbial associations through selection of varieties that more readily associate with beneficial microbiomes. In this paper current knowledge of the factors that drive N availability in cereal crops and how varieties would differ in terms of accessing N from soil is discussed.
Decomposition, mineralisation and N supply potential
Crop residues are a major source of carbon (C) for soil biota in the low organic matter Australian agricultural soils, hence retention of stubble is necessary for maintaining biological activity and N cycling within the cropping system. Decomposition of crop residues is carried out by diverse groups of microbial communities and facilitated by the activity of soil fauna. An increased practice of intensive cropping, especially cereals, away from mixed farms where crop rotation with legume pastures was common, has generally resulted in a decline of crop residue based N mineralisation mainly through altered crop residue quality e.g. high C:N ratio of cereal residues (100:1) replacing N-rich legume residues with C:N ratio between 15 and 25. Crop residues with a C:N ratio >22:1 generally result in immobilisation (tie-up) of mineral N in MB. Decomposing legume crop residues make a significant contribution to the N requirement of the following cereal crops, for example an apparent recovery of 30±10% of legume residue N by following wheat crops was observed over 20 legume treatments in dryland experiments conducted in eastern Australia (Peoples et al. 2017). Cereal stubble is not a major source of N for following cereal crops but should mainly be seen as a source of C for microbes. In no-till systems, only 1% to 6% of the N requirement of cereal crops is derived from the previous year’s wheat stubble (Gupta 2016 and GRDC project CSP00186). Non-cereal break-crops (e.g. legumes and canola) also help cereals in rotation to better access the soil mineral N pool through improved root health by reducing cereal root diseases (Gupta et al. 2011).
The rate and timing of N mineralisation regulates plant available mineral N in soils and the release of mineral N in soil is regulated by the microbial turnover processes (Figure 1). Changes in the amount of MB (a store-house for nutrients in soil) due to management, seasonal conditions and the rhizosphere effect, can exert a substantial impact on net N mineralisation and microbial immobilisation. MB-C accounts for 1.5% to 3.0% of soil organic C and MB-N for 2.0% to 5.0% of total N. In cereal crops, MB generally increases (by > 2-fold) from sowing to the end of flowering after which it reduces, depending upon seasonal conditions. Therefore, increasing MB and associated N tie-up would reduce N availability to cereals, especially during the early crop growth stages which should be supplemented with fertiliser N. Microbial activity and N cycling processes are generally higher in-crop due to the rhizosphere effect, e.g. approximately 60% of total soil microbial activity in-crop is concentrated in the rhizosphere, and hence a growing cereal crop has a significant impact on N mineralisation and immobilisation. Recent research has shown that MB and activity based drivers of N availability (mineralisation-immobilisation) vary between cereal crops and varieties. For example, rhizosphere MB and activity within a crop is significantly different between cereal crops e.g. cereal rye>oats>barley>wheat=triticale. Also, the amount of MB and activity in the rhizosphere of wheat varieties significantly varied in crops at Roseworthy in 2018, e.g. Wyalkatchem and Janz supported higher levels compared to Yitpi, Scepter and Machete varieties (Gupta and Sadras, 2018).
As the N uptake by wheat is 35% to 45% from the fertiliser in the year of application and the remainder from soil processes, a better prediction of in-crop N supply from soil for cereal crops is potentially important. As microbial turnover and associated N mineralisation-immobilisation balance is influenced by seasonal conditions, estimation of N supply potential at the beginning of a crop season should include the amount of N in MB and the N that can be mineralised from SOM and crop residues. N tie-up within a crop is only a temporary constraint as the immobilised N will be released through microbial turnover, typically in spring provided there is sufficient rainfall to maintain topsoil moisture. N tie-up by cereal residue not only occurs following incorporation but also happens in surface-retained and standing-stubble systems particularly pre-sowing depending upon season conditions. The extent to which differences emerge are related to seasonal conditions; chiefly rainfall and temperature, and to the time period between stubble treatment (burning or grazing) and soil sampling to allow differences to develop.
Residual fertiliser N use efficiency refers to the amount of N applied to a previous crop which becomes available to the following crop. Results from three years of field experiments at Karoonda (SA), Horsham (Vic) and Temora (NSW) indicated that the uptake of residual urea fertiliser applied to a wheat by the following wheat crops was 4% to 10% and 2% to 13% in the second and third year after application, respectively (GRDC project CSP00186). When considering the residual benefit of N fertiliser applied in 2018 to the 2019 cereal crop, crop performance and the influence of microbial processes should be considered.
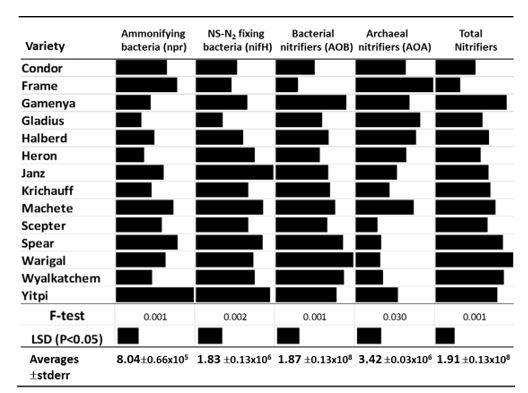
Figure 2. Abundances of microbial functional groups (gene copy number/gram soil) that regulate different N cycling processes in the rhizosphere of wheat varieties in a field experiment on a red brown earth soil at Roseworthy, SA during 2018 crop season (Gupta and Sadras, unpublished, SAGIT funded project).
Nitrification
Nitrification, the biological process of conversion of ammonium N (NH4+) to nitrate (NO3-), is an important step in the pathways that lead to releasing plant available mineral N (mineralisation, fertiliser release) and also losses of N from agricultural systems. Ammonia oxidation is carried out by several groups of microorganisms known as ammonia oxidising bacteria (AOB, bacteria amoA) and ammonia oxidising archaea (AOA, archaea amoA) collectively known as nitrifiers. Nitrifiers are generally abundant in Australian agricultural soils, although the abundance and the type of nitrifiers present varies with soil type and depth. Their activity can be influenced by management practices such as tillage, agrochemical application and crop type. Recent research has shown significant variation in the total nitrifiers, proportion of AOB and AOA and nitrification in the rhizosphere of different wheat varieties (Figure 2). AOA are generally higher in soils with constraints e.g. highly alkaline soils. Although the nitrification potential in most agricultural soils is not considered a limiting factor for the overall N mineralisation, changes due to management and crop effects could impact on the rate of activity and timing of N mineralisation. Research has shown that banding fertilisers can influence nitrification and the accumulation of nitrate N (Angus et al. 2014). A number of manufactured compounds are known to inhibit nitrification (such as nitrapyrin, dicyandiamide, 3, 4- dimethylpyrazole phosphate-DMPP) including some herbicides (e.g. triazines) thereby reducing the rate of mineral N release.
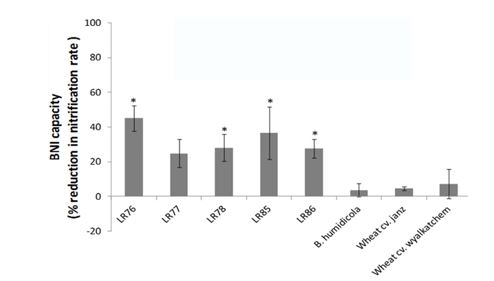
Figure 3. Biological nitrification inhibition (BNI) capacity of selected landraces of wheat in a pot assay. * indicates significant BNI at P<0.05 (O’Sullivan et al. 2016).
Biological nitrification inhibition (BNI), the production of nitrification inhibitors in situ by plant roots, is an attractive low-cost alternative to the application of commercial inhibitors. BNI compounds in root exudates affect both AOB and AOA microbial groups. Recent research has shown significant levels of BNI in Triticum aestivum; of the 96 wheat landraces tested, 26 produced root exudates which caused a statistically significant reduction in nitrification rates suggesting that some plants can moderate the rate of nitrate N release (Figure 3). The discovery of landraces with BNI ability and the presence of moderate levels in the wheat cultivar Wyalkatchem raises the potential for breeding this trait into modern, elite wheat cultivars. At present, fertiliser N use efficiency in cereal crops could be manipulated by targeting fertiliser placement or by using slow release fertilisers and nitrification inhibitors.
Non-symbiotic free-living, N2 fixation
Free living (FL) N2 fixation refers to N fixation by bacteria growing independently in soil and stubble or in close association with plant roots. With the increased adoption of intensive cropping and the corresponding increase in area under consecutive cereal crops (>50%), FL-N2 fixation has the potential to make substantial contribution to N requirements in cereal crops. In cereal crops under current day conservation farming systems, the soil habitat promotes activity of FL-N2 fixing (nifH gene harbouring) bacterial communities both during off-season and in crop i.e. increased microsites with C availability and high C/N ratio. Improvements in FL-N2 fixing capacity in cereals can provide multiple benefits through reduced requirement for N inputs, disease suppression and C sequestration. Estimates of FL-N2 fixation using soils from cereal fields measured using a laboratory assay, ranged from 0.2 to 1.5kg N/ha/day in sand and sandy loam soils in low to medium rainfall regions of southern and Western Australia, compared to 0.5 to 2kg N/ha/day in the clay and loam soils in high rainfall regions. The amount of N fixed varied with soil type (% clay content, pH) and was influenced by the time of sampling (in crop versus non-crop/fallow period), crop type, stubble retention and mineral N levels. The number of optimal days per season for FL N2 fixation does vary in different agricultural regions. Higher levels of mineral N in the surface soil (>25kg N/ha) could have a negative effect on the amount of FL-N2 fixation, but this varies with soil type so needs region-specific solutions.
Genetic profiling of N2-fixing bacteria (nifH gene sequencing analysis) in cereal crop field soils (from Qld, NSW, SA and WA) indicated that the presence of a diverse group of free-living community (112 genera) bacteria in different agricultural regions; indicating differences based on soil type, climate and management. Recent research has shown that cereal crops differed in terms abundance of N2 fixing bacterial populations and the amount of FL-N2 fixation they support in their rhizosphere soil and in roots e.g. barley=triticale >wheat.Additionally, there is some varietal based variation in the abundances of various groups affecting the amount of FL-N2 fixation. Among wheat varieties; Yitpi and Janz supported higher number of N2 fixing bacteria compared to Gladius and Frame (Figure 2). Barley varieties Flagship, Schooner showed higher abundance and higher FL N2 fixation compared to the variety Buloke and Hindmarsh. Varieties of wheat and barley also vary in the composition of diazotrophic bacterial community in their roots and rhizosphere soil, i.e. each cultivar preferentially supporting a narrow spectrum of microbes in higher abundance. The variation in FL N2 fixation with different cereal crops and the different varieties is therefore a product of the specific type of diazotrophs they promote and their abundance in roots and rhizosphere. Overall, results demonstrate the presence of a diverse community of N2 fixing bacteria in agricultural environments, and that the selection of appropriate cereal crop variety might increase N2 fixation benefits from FL N2 fixation especially during spring when the demand for N is high.
What next?
Recent research showed that selection for yield in SA wheat varieties reduced the size of the root system, but increased total N uptake (25% more N from soil) and N uptake per unit root system (Aziz et al. 2017). The reasons for the improved efficiency in uptake of N are not fully understood. The presence of a significant variation between cereal crops and varietal differences in general microbial composition and microbial functional traits (genes) involved in N availability raises the potential for breeding this trait into modern, elite cultivars of cereal crops. These traits could be used to promote mineralisation and free living N2 fixation during periods of peak N requirement. Varieties that can modulate the release of fertiliser N through higher biological nitrification inhibition would also help improve the fertiliser N use efficiency. Trade-offs between improved microbial activity and other crop traits need attention. In addition, the process of N immobilisation (tie-up) by the MB which affects the availability of N from soil organic matter and fertiliser early in the growing season should be considered in N management decisions, especially for cereal crops, in stubble retained and intensive cereal cropping systems.
Useful resources
Angus JF and Grace PR (2017) Nitrogen balance in Australia and nitrogen use efficiency on Australian farms. Soil Research. 55, 435-450.
Aziz et al. (2017) Five decades of selection for yield reduced root length density and increased nitrogen uptake per unit root length in Australian wheat varieties. Plant Soil. 413, 181-192.
Gupta, V.V.S.R. et al. (2011) Principles and management of soil biological factors for sustainable rainfed farming systems. In: Rainfed farming systems’ by P. Tow, et al., pp. 149-184, Springer Sci. & Business Media.
Gupta, V.V.S.R. (2016) Biological factors influence N mineralization from soil organic matter and crop residues in Australian cropping systems, Proc. of the 2016 International N Initiative Conference, "Solutions to improve nitrogen use efficiency for the world", 4 – 8 December 2016, Melbourne, Australia. http://www.ini2016.com/pdf-papers/INI2016_Vadakattu_Gupta.pdf
O’Sullivan C. et al. (2016) Identification of several wheat landraces with biological nitrification inhibition capacity. Plant Soil. 404, 61-74.
Peoples MB et al. (2017) Soil mineral nitrogen benefits from legumes and comparisons of the apparent recovery of legume fertiliser nitrogen by wheat. Soil Research. 55, 600-615.
Acknowledgements
The research undertaken as part of the different projects is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. Financial support for research on wheat varieties is provided by SAGIT and CSIRO. Stasia Kroker, Marcus Hicks, Bill Davoren, Willie Shoobridge at CSIRO and Mariano Cossani from SARDI provided technical support for laboratory and field work.
Contact details
Vadakattu Gupta
CSIRO Agriculture, Waite campus
08 8303 8579;
Gupta.Vadakattu@csiro.au;
@LifeinSoil5
Was this page helpful?
YOUR FEEDBACK