Feathertop Rhodes Grass (Chloris virgata): Seed ecology and Herbicide Resistance Status of a New Weed Threat to Western Australia
Author: Alexandra Douglas and Abul Hashem, DPIRD | Date: 26 Feb 2019
Key Messages
- Feathertop Rhodes grass (FTRG) is already widespread along the sides of many of Western Australia’s major country roads.
- Studies on FTRG seed survival have shown up to 96% of the seeds germinated when tested at three and six months after burial at three depths. This high level of germination indicates that there may be limited dormancy of FTRG in the field and that there may be a good chance of eradicating FTRG populations should they invade paddocks from the roadside.
- Some FTRG populations collected from roadsides in the WA grain-belt have been found resistant to paraquat + diquat and glyphosate. This is of concern, as these herbicides are commonly applied to roadside vegetation.
Aims
Feathertop Rhodes grass (FTRG, Chloris virgata) is a warm-season, annual grass which is a major weed in the northern cropping region of Australia and is now being reported in the southern and western regions (Borger et al, 2018). It is closely related to native Windmill grass (Chloris truncata) but when mature, they are easily distinguished by their seed heads. FTRG germinates and develops rapidly following summer rainfall. Seeds are small and spread through wind and water. One FTRG plant can produce up to 40,000 seeds and seeds can be produced over a number of weeks if growing conditions are favourable (Ngo et al 2017). Five populations of this species have been confirmed resistant to glyphosate in Queensland, New South Wales and South Australia (Heap 2019). In Western Australia, FTRG has been found to invade paddocks following flood events when seed and soil have been moved into paddocks from roadside stands. Roadside weed populations are most commonly sprayed with glyphosate and paraquat. Since this weed has natural tolerance to glyphosate and paraquat, tolerant biotypes from the roadside may move into crops within WA. There is little information on the biology and resistance status of this species in WA, which makes it difficult to develop management strategies.
The aim of this research was 1) to investigate the seed ecology of FTRG populations and 2) to determine the levels of resistance to glyphosate and paraquat.
Method
Experiment 1 - Factors affecting germination, seed burial
A trial was established at Northam to determine the effect of depth of seed burial on the persistence of FTRG. Fifty seeds from each of three FTRG populations were placed in nylon bags and buried at different depths (0, 2, and 10cm) in June 2017. Bags were collected in September and December 2017 and June 2018, further collections are planned at 24 and 36 months after burial.
Following collection, seeds were germinated by removing from the bags and placed in petri dishes with distilled water and gibberellic acid (0.1g/1L solution). FTRG seeds were subjected to a 12 hour, 20/30°C light/dark cycle. Dishes were checked 10 days after the test commenced, germinated seedlings were counted and removed and the test continued for a further 10 days, when the final count was made. The percentage germination was calculated from total number of seeds initially placed in the bags at the time of burial. In the 0 and 2cm depths, a proportion of seeds had germinated and died/rotted prior to recovery these were included in calculating the percentage germination reported in Table 1.
Experiment 2 – Herbicide resistance
Experiment 2 was conducted from April to May 2018 on 11 populations collected in 2016 from the roadside of WA, in a glasshouse at DPIRD, Northam. These populations were different to those used in Experiment 1. Clean seed of each population was initially stored in a non-air conditioned glasshouse to allow after-ripening, and then at room temperature until 2018. Plastic trays (34cm long x 12cm wide x 5cm deep) were filled to within 2cm of the top with potting mix. Plastic dividers were used to separate each tray into three sections. Seeds of each population (20-40 per population per section of tray) were sown on the surface of the potting mix under dry conditions on 3 April 2018. Seeds were lightly pressed into the potting mix to minimise movement by air conditioning wind flow. The plastic trays were placed in an aluminium tray lined with thick plastic. After sowing, the aluminium tray was filled with water so the potting mix in the plastic tray could absorb water. The experiment was laidout in a completely randomised block design with three replications.
Seedlings were thinned to 10-12 plants per plot on 26 April 2018. When the seedlings were at 3-4-leaf stage, trays were sprayed with herbicides on 10 May 2018. Treatments included Roundup Power MAX® (glyphosate 540 g ai/L) at 0, 270, 540 and 1080g ai/ha, and Spray.Seed® (paraquat 135 g ai/L + diquat 115 g ai/L) at 0, 250, 500 and 1000g ai/ha. Final assessment of plant survival was recorded on 26 May 2018, which was 16 days after spraying (16 DAS).
Any surviving plants were maintained for seed production. Mature progeny seed of each population was harvested, bulked across replications and stored to be used in Experiment 3.
Experiment 3 – Herbicide resistance in progeny
Experiment 3 was conducted from 15 November 2018 to 15 January 2019 on the progenies of the populations surviving treatments in Experiment 2. The plants were grown and tested for resistance following the same procedures as Experiment 2. However, an additional treatment was used with 2160 g ai/ha of glyphosate and 2000g ai/ha of paraquat + diquat. Seed was sown on 19 November 2018 and herbicide treatments were applied on 18 December 2018. Visual assessment of seedling survival was made on 11 January 2019 (23 DAS). The results of this experiment will focus on two of the 11 populations tested (population 49 and 159, which demonstrated the highest levels of tolerance).
Results
Experiment 1 - Factors affecting germination, seed burial
When seed was tested at three and six months, all populations had very high germination (Table 1). By 12 months, there was greater variation between the populations. The Mukinbudin population had very low germination of seeds collected from any depth. The other two populations had low germination from the surface seed, but greater germination from seed at 2 to 10cm.
Table 1. Germination (%) of feathertop Rhodes grass seeds collected following burial at various depths for 3, 6 and 12 months, for three populations. Lsd values indicate the differences in means where P values were significant. Values followed by the same letter, within the same collection period, are not significantly different.
Population | 3 month | 6 month | 12 month | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0cm | 2cm | 10cm | 0cm | 2cm | 10cm | 0cm | 2cm | 10cm |
Corrigin/Quairading | 64.7 | 89.3 | 84.0 | 96.0e | 90.7de | 70.0bc | 9.3a | 44.0b | 37.3b |
Arthur River | 78.7 | 84.7 | 78.7 | 73.3bc | 87.3de | 67.3bc | 7.3a | 48.0b | 49.3b |
North Mukinbudin | 74.0 | 91.3 | 74.7 | 31.3a | 78.7cd | 65.3b | 0.0a | 0.0a | 7.3a |
Average over population | 72.4 | 88.7 | 79.1 | 66.9 | 85.6 | 67.6 | 5.5 | 30.6 | 31.3 |
5% Lsd (depth) | ns | ns | 17.4 | ||||||
5% Lsd (population) | ns | 13.4 | 17.4 | ||||||
Experiment 2
In Experiment 2, populations 49 and 159 had a high level of tolerance to both glyphosate and paraquat + diquat. Plants in other populations were initially affected by glyphosate but most plants survived and continued to grow. Some plants in some populations initially appeared dead but continued to reshoot from the base. A small proportion (3 to 15%) of plants in populations 42, 49 and 159 survived 540 and 1080g ai/ha of glyphosate (Figure 1, top). Similarly, eight per cent of plants in populations 45, 49 survived up to double the label rate of paraquat + diquat (Figure 1, bottom). Only population 157 was completely susceptible to glyphosate and to paraquat + diquat (Figure 1).
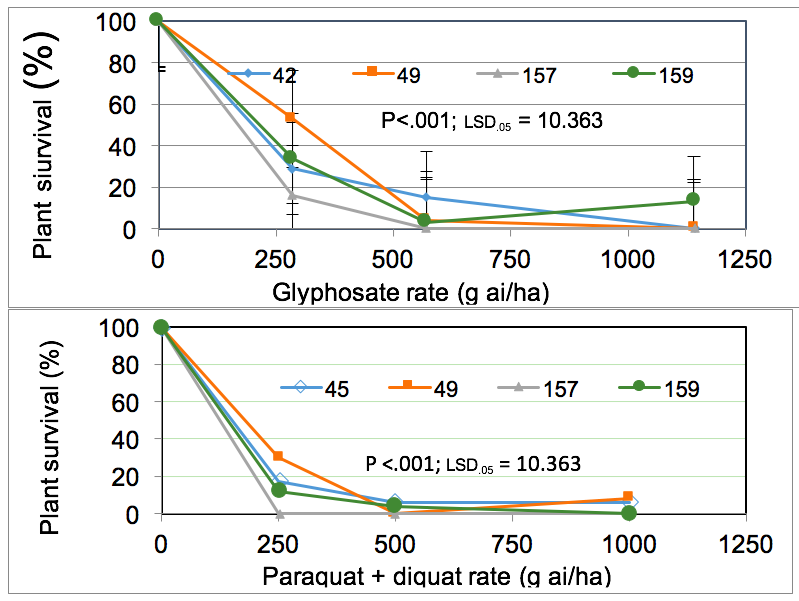 Figure 1. Plant survival (%) of four populations of feathertop Rhodes grass to various rates of glyphosate (g/ha) and paraquat + diquat (g/ha) 16 days after spraying at Northam 2018 in Experiment 2.
Figure 1. Plant survival (%) of four populations of feathertop Rhodes grass to various rates of glyphosate (g/ha) and paraquat + diquat (g/ha) 16 days after spraying at Northam 2018 in Experiment 2.
Experiment 3
On average, the progeny of populations 49 and 159 had 100% survival at 270 g ai/ha of glyphosate and 500g ai/ha of paraquat + diquat (Figure 2). Average survival of these populations was 80%, 75% and 50% at 540, 1080 and 2160g ai/ha of glyphosate, and 58% to 20% at 1000 to 2000gai/ha of paraquat + diquat (Figure 2).
These results suggest that these two populations are likely to be resistant to both glyphosate and paraquat + diquat. To confirm the intensity of resistance, calculation of an LD50 ratio is required.
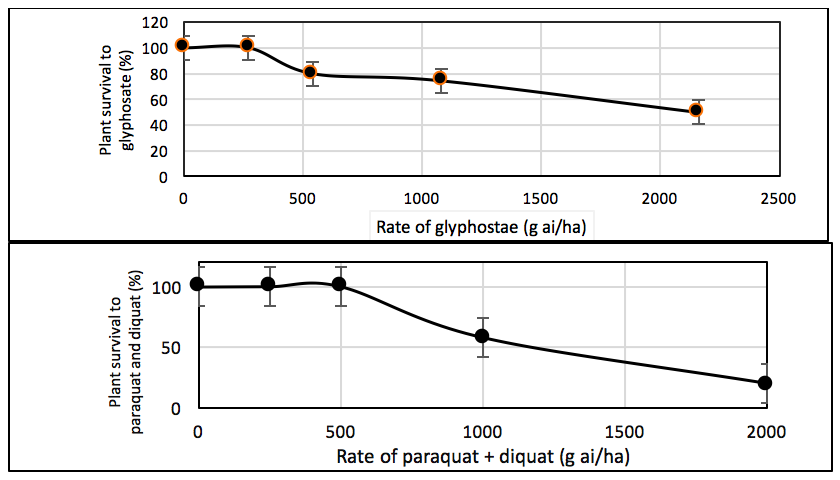 Figure 2. Survival of progeny plants of two feathertop Rhodes grass populations (Populations 49 and 159) to various rates of glyphosate or paraquat + diquat 23 days after spraying in Experiment 3 at Northam 2018. An average of the two populations is presented here. Vertical bars represent the standard error of the mean of four replications.
Figure 2. Survival of progeny plants of two feathertop Rhodes grass populations (Populations 49 and 159) to various rates of glyphosate or paraquat + diquat 23 days after spraying in Experiment 3 at Northam 2018. An average of the two populations is presented here. Vertical bars represent the standard error of the mean of four replications.
Conclusion
The high levels of germination from the three and six month collections indicates that there may be limited dormancy of FTRG in the field. The low germination of surface seed at twelve months indicates that seed left on the soil surface will degrade. However, the seed buried at 2 or 10cm did not degrade after 12 months. Therefore, this weed will be easier to eradicate when seed is left on the soil surface, as in a pasture or zero-tillage system. Buried seed will form a seed bank that will likely persist for at least a year. However, further measurements are required to understand longevity over this time frame
Grazing by livestock provides a viable non-chemical control option. This will be valuable because of the natural tolerance and resistance to non-selective herbicides observed in FTRG. Two populations of FTRG collected from WA roadsides have developed a high level of tolerance (probably resistance) to both glyphosate and paraquat + diquat. However, the progeny plants of these populations appear to be more tolerant to glyphosate than paraquat + diquat. This weed species has already developed resistance to glyphosate in Queensland, New South Wales and South Australia. After developing resistance, the resistant biotypes of this species may be more likely to move into crops as they develop tolerance to the current non-selective herbicide molecules. This will require alternative management options with herbicides from different mode of action.
References
Borger, C., Hashem, A., Amjad, M., Wild, C. Nicholson, D., Douglas, A., Peltzer, S., Swift, B., Rossi, A., Delroy, J. Cockburn, E., Butler, A., Clarke, E., Chamber, K. and D’Anutono, M. (2018) Summer weeds within the Western Australian wheatbelt – a GRDC survey. In: Grains Research Updates. Grains industry Association of Western Australia, Perth, Western Australia.
Heap, I (2019) International survey of herbicide resistance. Available at: Weed Science
Ngo, T.D.; Boutsalis, P.; Preston, C.; Gill, G. (2017) Growth, Development, and Seed Biology of Feather Fingergrass (Chloris virgata) in Southern Australia. Weed Science 65(3):413-425.
Acknowledgments
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and GRDC investment, the author would like to thank them for their continued support. Dave Nicholson and Nerys Wilkins for excellent technical support. Thanks also to Dr Catherine Borger for reviewing the paper.
Paper reviewed by: Dr Catherine Borger
GRDC Project Number: DAW00257
GRDC Project Code: DAW00257,
Was this page helpful?
YOUR FEEDBACK
