Soil pore water chemistry explains improvement in root growth of grain crops better than 0.01 M CaCl2 extract in an acidic soil from Kalannie, WA
Author: Gaus Azam, Chris Gazey and Mario D'Antuono, Department of Primary Industries and Regional Development. Northam, Western Australia | Date: 25 Feb 2019
Key Messages
- Measurements of pH and Al in the soil solution (soil pore water) showed a significant interaction of lime x gypsum but such effects were not apparent in CaCl2 extract. Al concentration in subsurface soil solution showed stronger relationship (R2=0.93, P ≤0.001) with total root length for all crop species than Al concentration in CaCl2 extract (R2=0.73, P ≤0.001).
- Root growth was less responsive to liming in a 50:50 mixture of top- and subsoil (mixed soil), than in 100% subsurface soil. In mixed soil canola roots showed the highest response to liming, but in subsurface soil barley showed the highest response to liming, indicating that a barley crop might produce a greater yield advantage if subsurface soil acidity is corrected to the target level.
- The application of lime and gypsum together increased root length of all three crops more than the application of either ameliorant individually, indicating that the combined application of lime and gypsum may allow access to subsurface soil moisture below the seedbed.
Aims
Lime and gypsum are used to ameliorate acidic soil (Li et al. 2019; Easton, 2015). The effects of lime and gypsum application on grain yield has also been reported extensively (Conyers and Scott, 1989; Smith et al., 1994; Sumner et al., 1986), but effects on crop establishment and early root growth have not, especially for Western Australian conditions.
In acidic soil improvement in soil solution (i.e. soil pore water) pH and a decrease in aluminium (Al) concentration, due to lime and gypsum application, are significant for crop establishment with the additional aim to produce deeper root systems. This can be more relevant in south west of Western Australia as grain growers are adopting dry seeding (Fletcher et al., 2016). Under dry soil conditions, the constituents in the soil solution become more concentrated and hence the effect of soil solution pH and Al chemistry on early plant growth can be multiplied (Rhoades et al., 1976). Whitten (2002) has suggested that soil pH measured in the soil solution has a stronger correlation with crop yield than the soil pH measured in 1:5 soil/water or soil/CaCl2 suspension. However, there is limited research which compares the efficiency of measuring pH and Al concentration in soil solution and 1:5 soil/water or soil/CaCl2 suspension to explain the improvement in early root growth of different grain crops.
This paper reports the outcomes of a glasshouse experiment that was designed to examine a) the effects of lime and gypsum addition on soil solution chemistry (using two different extraction methods) and b) their impact on early root growth of wheat, barley and canola, three widely cultivated crops in Western Australia. The relationships between soil solution chemistry and plant growth were also examined.
Method
Bulk surface, SF, (0-10 cm) and subsurface, SS, (20-40 cm) soil were collected separately from a continuously cropped paddock near Kalannie, Western Australia (35°42’S, 117°29’E). This soil is classified as a yellow-orthic Tenosol in the Australian Soil Classification. The soil was air-dried and sieved though a 2 mm mesh before using in the experiment. The SF and SS soil had similar particle size distribution (87% sand, 3% silt and 10% clay). Both surface and subsurface soil was strongly acidic and, particularly the subsoil, extremely aluminium toxic. The soil had low levels of organic carbon. N, P and K contents were in average level in the topsoil, however, subsoil had very limited level of Colwell P which also had very very low phosphorus buffering index (Table 1).
The experiment had a complete factorial design with four lime rates, two gypsum rates and two types of soil. The lime treatments were: 0 (L0), 0.313 (L1), 0.625 (L2) and 1.250 (L3) t ha-1 lime (neutralising value of 94.9%, 99.2% particles <0.5 mm)) when mixed to 10 cm soil depth. Two gypsum (gypsum purity 96%) levels were used: 0 (G0) and 0.625 (G2) t ha-1 when mixed to 10 cm soil depth. The two soil types were the SS and a 50:50 mixture of surface- and subsoil (mixed soil, MS). There were three replications for all treatments.
The lime and gypsum treatments were applied separately. After mixing soils with lime treatments, 800 g of treated soil was packed into a tapered pot (953 mL capacity, 10 cm high, 12 cm top diameter, 10 cm basal diameter). The gypsum treatments were applied to the surface of the pots. Water was added to pots to reach field capacity (16% volumetric water content). All the pots were then incubated for seven days.
Three widely cultivated crops in Western Australia: barley (Hordeum vulgare cv. La Trobe), wheat (Triticum aestivum cv. Mace) and canola (Brassica napus cv. hybrid Hyola 559TT) were tested. Five seeds were sown at 3 cm depth in each pot and placed in a growth room (26 ± 2 °C temperature, 60 ± 10% relative humidity, 14 h photoperiod) for germination for five days. Pots were then moved to a controlled glasshouse. After a week in the glasshouse, a 5 mm layer of polyethylene beads (AlkatheneTM) was applied to the surface of each pot to minimise evaporation. Pots were weighed and watered every 2-3 days to maintain field capacity. No drainage from the pots was observed.
Table 1. Properties of surface- and subsurface soil used in the experiments
Parameters | Surface soil | Subsurface soil |
|---|---|---|
Sampling depth (cm) | 0-10 | 20-40 |
Texture | Sandy loam | Sandy loam |
Bulk density (g/cm3) | 1.34 | 1.52 |
pHCa | 4.35 | 3.95 |
NH4-N (mg kg-1) | 7.33 | 2.33 |
NO3-N (mg kg-1) | 11.00 | 6.33 |
Colwell P (mg kg-1) | 31.3 | 4.33 |
PBI | 24.2 | 63.1 |
Colwell K (mg kg-1) | 53.3 | 32.3 |
OC (%) | 0.85 | 0.32 |
S (mg kg-1) | 7.4 | 28.6 |
CEC (meq 100 g-1) | 1.51 | 1.09 |
AlCa (mg kg-1) | 2.47 | 22.2 |
Soil solution was collected from all pots at 28 days after sowing. The solution was extracted using a rhizon sampler (MOM; porous tube, 5 cm long and 2.5 mm diameter; mean pore size of 0.15 µm; Rhizosphere Research Product, Wageningen, the Netherlands). Rhizon tube was placed at the center of the pot and between 2.5-7.5 cm soil depths. The solution samples were preserved at 4°C until the pH and aluminium concentrations were measured.
Plants were harvested at 33 days after emergence. At the harvest a 100 g subsample of soil was collected from the center (from where soil solution was collected) of each pot. The subsamples were dried at 40oC in a forced-drought oven and passed through a 2 mm sieve before chemical analysis. Soil pH in 1:5 soil:CaCl2 (0.01 M) extract (Method 4B1, Rayment and Lyons 2011) and CaCl2-extractable total Al (Bromfield, 1987) were measured. After the subsample was extracted, roots were separated from the remaining soil using a gentle jet of water over a 0.5 mm sieve. All roots from each pot were then scanned using a high-resolution (600 dpi) scanner. Total root lengths were measured with WinRhizo software (v. 2005c; Régent Instruments Inc., Quebec, Canada).
A linear model was fitted to each of the measurements, i.e., root length, soil pH and Al concentration using the ANOVA procedure in GenStat (Version 18.1, VSN International, Oxford, UK) to compare the factorial treatments of lime x gypsum x soil type with polynomial contrasts. For non-normal data, a log-transformation (log10) was performed to stabilise the variance. Fisher's protected least significant difference (LSD) was applied at the 0.05 significance level. Linear regression was performed between soil parameters (pH and Al concentration) and total root length. When reporting treatment effects, they were ordered by the relative (%) change between the lowest and the highest.
Results
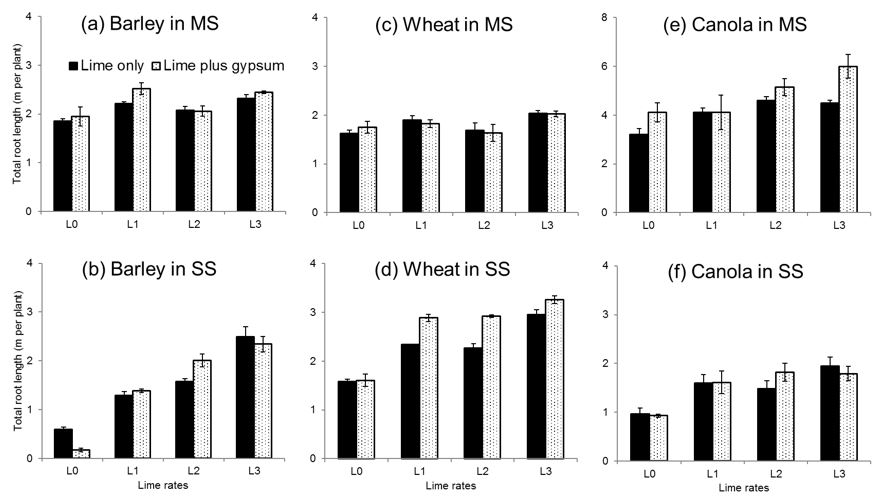
The interaction of lime x gypsum x soil type to improve root length was significant (P ˂ 0.05) for barley and wheat crops but not for canola (Fig. 1). The differences we observed in treatment effects are mainly in fine roots (data not presented). Treatment effects for each crop species are described separately below.
For barley, the magnitude of the main treatment effect was in the order of lime level > soil type > gypsum level. The influence of increasing lime on root length was more prominent in SS (Fig. 1b; 144% increase in L3) than in MS (Fig. 1a; 17% increase in L1). Barley seedlings produced longer root length in MS (2.18 m per plant) than in SS (1.48 m per plant). The main effect of gypsum application had no significant (P ≥0.05) influence on root length, however, a negative effect of gypsum was observed when applied in SS (Fig. 1b). The significant interaction of lime level x gypsum level x soil type occurred when G2 was applied with L1 in MS (Fig. 1a) and with L2 in SS (Fig. 1b).
For wheat, the magnitude of main treatment effect was also in the order of lime level > soil type > gypsum level. Again, liming improved wheat root length at higher magnitude in SS (Fig. 1d; 100% increase in L3) than in MS (Fig. 1c; 25% increase in L3). The main effect of soil type on root length of wheat was also significant (P ˂ 0.05) but it was opposite to that of barley. Wheat grew longer root length (2.48 m per plant) in SS than in MS (1.81 m per plant). The main effect of gypsum was significant (P ˂ 0.05) as well where gypsum application produced longer root length of wheat (2.24 m per plant) than in untreated control (2.05 m per plant). For wheat, the significant (P ˂ 0.05) interaction of lime level x gypsum level x soil type occurred only in SS when G2 was applied with L1 or greater level of lime (Fig. 1d).
Figure 1: Response of barley, wheat and canola root length to incorporation of lime at different rates with and without gypsum in a subsoil and 50:50 mixture of top- and subsoil from Kalannie, WA. Vertical bars represent ± standard error of the mean root length. Scales on Y-axes are different due to varying responses from crop types. Statistics: LSD of means root length (5% level) for soil type x lime x gypsum (P<0.05): barley = 0.30, wheat = 0.27 and canola = 0.84.
For canola, the response was different to the cereals; the magnitude of main treatment effect was in the order of soil type > lime > gypsum and there was no significant interaction between treatments. Canola produced significantly longer root length when it was grown in MS (4.47 m per plant) than in SS (1.52 m per plant). Application of increasing lime level had a significant effect on root length of canola as we observed for the other two crops. Overall with the application of L3 there was 43% and 97% increase in root length for MS (Fig. 1e) and SS (Fig. 1f), respectively. Application of gypsum also increased canola root length (3.19 m per plant) in MS compared to the untreated control (2.80 m per plant).
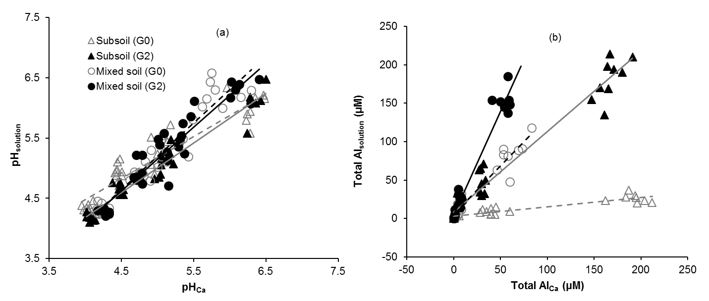
Regression analysis showed that there was a strong relationship (R2 = 0.91-0.97; P≤0.001) between soil pH measured in soil solution and CaCl2 (Fig. 2a). There was also a strong relationship (R2 = 0.85-0.96; P≤0.001) between soil Al concentration measured in two extraction methods (Fig. 2b). Overall, solution pH was only 3% higher compared to the pH measured in CaCl2 suspension (Fig. 2a) but there was large difference in soil Al (Fig. 2b) measured in two methods. The effect of extraction method on soil Al depended on soil type; soil Al was 7.6 times higher in CaCl2 compared to soil solution from lime treated SS, whereas it was 2.8 times lower in CaCl2 compared to Al concentration in soil solution collected from lime and gypsum treated MS (Fig. 2b).
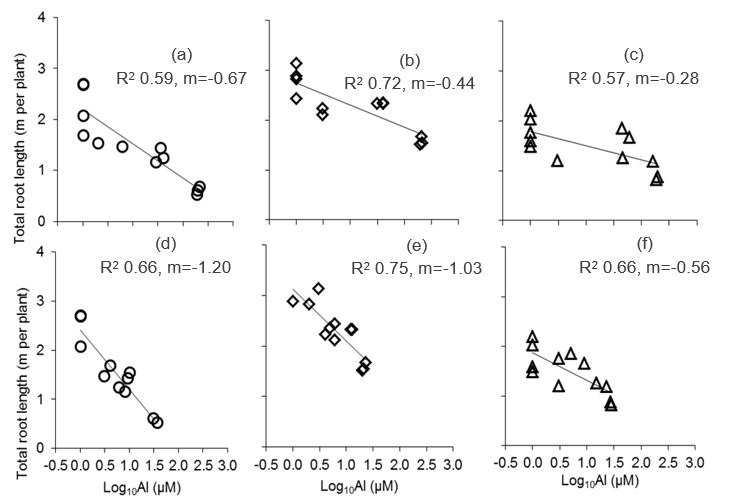
Root length of all three crops grown in subsoil were strongly related (R2 = 0.57-0.75; P≤0.001) with soil Log10Al concentration (Fig. 3). However, root length was more strongly related with the Al concentration in soil solution (Fig. 3d, 3e and 3f; R2 = 0.66-0.75; P≤0.001) than in CaCl2 suspension (Fig. 3a, 3b and 3c; R2 = 0.57-0.72; P≤0.001). Among the three crops barley (Fig. 3a and 3d) had the highest and canola had the lowest slope, respectively (Fig. 3c and 3f).
Figure 2: Relationship between soil pH (a) and Al (b) measured in 0.01 M CaCl2 extract as well as in soil solution (collected at field capacity). Grey and black dotted lines represent regression lines for the lime treated subsoil and mixed soils, respectively. While grey and black solid lines represent regression lines for the lime plus gypsum treated subsoil and mixed soils, respectively. Statistics: n = 12, regression coefficient significant at P≤0.001.
Figure 3: Relationship between Al concentration in subsoil, measured in 0.01 M CaCl2 extract (a, b and c) or soil solution (d, e and f), and root length of barley (a and d), wheat (b and e) and canola (c and f). Statistics: n = 12, regression coefficient significant at P≤0.001.
Conclusion
Suitability of extraction method
A strong relationship was found between soil pH measured in 0.01 M CaCl2 suspension and soil solution where soil solution pH was almost equal to the pH measured in CaCl2 suspension. This finding suggests that there is no need to use a conversion factor in relating soil solution pH to the corresponding soil pH measured in 1:5 soil/CaCl2 suspension as suggested by Ahern et al., (1995), but this needs further validation for a wider range of soil types.
Measuring Al in soil solution identified a bimodal lime x gypsum x soil type interaction but measurements from CaCl2 suspension failed to do so. We found CaCl2 suspension overestimated Al for subsurface soil treated with lime only. This is probably because the CaCl2 extract is too strong for low ionic strength subsurface soil and hence over estimated Al from the exchange sites (Carr et al., 1991). Carr et al. (1991) also recommended the use of low strength extractants such as 0.005 M KCl especially for soils with low ionic strength. More importantly our data showed that the Al concentration in the subsurface soil solution showed a stronger relationship (overall R2=0.93) with total root length than Al concentration in CaCl2 extract (overall R2=0.73). This is in agreement with Whitten (2002) who has reported that soil pH measured in soil solution is strongly correlated with crop growth and yield.
Crop type response to lime and gypsum
Results showed that increasing lime level increased root length of barley, wheat and canola. Barley was the most responsive crop to subsurface soil liming followed by wheat and canola. This is because barley is more responsive to decreased Al toxicity in subsurface soil than wheat or canola (Tang et al., 2003). The differences we observed in treatment effects were mainly in fine roots, due the differences in Al tolerance for different root branch orders. At higher levels of toxic Al, seminal roots are less affected compared to the fine roots and the root hairs (Delhaize and Ryan 1995). We observed combined application of lime and gypsum was more efficient to increase root length. Lime increased soil pH and decreased toxic Al; gypsum on the other hand might have reduced the toxicity of Al without decreasing the relative amount of Al compared to other cations in the soil solution (Rengel, 1992).
Effect of soil type
We found liming had similar effects in mixed soil and subsurface soil on improving soil pH and decreasing Al concentration but root growth was not greatly improved in ameliorated MS compared to the untreated MS, especially for the two cereal crops. Mixing topsoil with considerably higher organic carbon with inorganic subsurface soil redistributed the carbon pool which is likely to have bound with the toxic aluminium and converted it into less toxic forms of Al (Wong and Swift, 2003). Such mixing might also provide an additional benefit for crop nutrition, such as improvement in phosphorus uptake (Scanlan et al., 2017).
Acknowledgments
The research undertaken as part of GRDC invested project DAW00252, the author would like to thank them for their continued support. We would also like to thank staff at the Department of Primary Industries and Regional Development at Northam.
GRDC Project Number: DAW00252
Paper reviewed by: James Fisher
References
Ahern, C. R., Baker, D. E., and Aitken, R. L. (1995). Models for relating pH measurements in water and calcium chloride for a wide range of pH, soil types and depths. In "Plant-Soil Interactions at Low pH: Principles and Management: Proceedings of the Third International Symposium on Plant-Soil Interactions at Low pH, Brisbane, Queensland, Australia, 12–16 September 1993" (R. A. Date, N. J. Grundon, G. E. Rayment and M. E. Probert, eds.), pp. 99-104. Springer Netherlands, Dordrecht.
Carr, S.J., Ritchie, G.S.P., and Porter W.M. (1991). A soil test for aluminium toxicity in acidic subsoils of yellow earths in Western Australia. Australian Journal of Agricultural Research 42, 875-892.
Delhaize, E., and Ryan, P. R. (1995). Aluminum Toxicity and Tolerance in Plants. Plant Physiology 107, 315-321.
Easton, J. (2015). Soil Acidity – Can Gypsum Increase Wheat Yields on Aluminium Toxic Soils? Research Update: Grain Research and Development Corporation, Perth.
Fletcher, A., Lawes, R., and Weeks, C. (2016). Crop area increases drive earlier and dry sowing in Western Australia: implications for farming systems. Crop and Pasture Science 67, 1268-1280.
Li, G.D, Conyers, M.K., Heylar, K.R., Lisle, C.J. and Poile, G.J. (2019). Cullis, Brian R.Long-term surface application of lime ameliorates subsurface soil acidity in the mixed farming zone of south-eastern Australia. Geodarma 338, 236-246.
Rayment, G., and Lyons, D. (2011). Soil chemical methods: Australasia. CSIRO Publishing, Melbourne.
Rengel, Z. (1992). Role of calcium in aluminium toxicity. New Phytologist 121, 499-513.
Rhoades, J. D., Raats, P. A. C., and Prather, R. J. (1976). Effects of Liquid-phase Electrical Conductivity, Water Content, and Surface Conductivity on Bulk Soil Electrical Conductivity1. Soil Science Society of America Journal 40, 651-655.
Scanlan, C. A., Brennan, R. F., D'Antuono, M. F., and Sarre, G. A. (2017). The interaction between soil pH and phosphorus for wheat yield and the impact of lime-induced changes to soil aluminium and potassium. Soil Research 55, 341-353.
Smith, C., Peoples, M., Keerthisinghe, G., James, T., Garden, D., and Tuomi, S. (1994). Effect of surface applications of lime, gypsum and phosphogypsum on the alleviating of surface and subsurface acidity in a soil under pasture. Soil Research 32, 995-1008.
Sumner, M. E., Shahandeh, H., Bouton, J., and Hammel, J. (1986). Amelioration of an Acid Soil Profile through Deep Liming and Surface Application of Gypsum. Soil Science Society of America Journal 50, 1254-1258.
Tang, C., Rengel, Z., Diatloff, E., and Gazey, C. (2003). Responses of wheat and barley to liming on a sandy soil with subsoil acidity. Field Crops Research 80, 235-244.
Whitten, M. (2002). Amelioration and prevention of agriculturally generated subsurface acidity in sandy soils in Western Australia, University of Western Australia, Crawley, Australia.
Wong, M., and Swift, R. (2003). Role of Organic Matter in Alleviating Soil Acidity. In "Hand Book of Soil Acidity" (D. Rengel, ed.), pp. 337-358. Marcel Dekker Inc., Bsel, Switzerland.
GRDC Project Code: DAW00252,
Was this page helpful?
YOUR FEEDBACK