Soil solution concentrations and aluminium species of an eastern wheatbelt acidic soil of WA treated with lime and gypsum.
Author: Geoff Anderson DPIRD, Shahab Pathan DPIRD, Rajesh Sharma ChemCentre, David Hall DPIRD, James Easton CSBP | Date: 26 Feb 2019
Key Messages
- Both lime and gypsum applied to acidic soil have led to long term improvement in wheat grain yield. Application of lime increases soil solution pH (pHSoln) and while the application of gypsum increases soil solution sulphate (SSoln).
- The increase in pHSoln converts toxic Al3+ to inert gibbsite while the increase in SSoln concentration results in the formation of inert aluminium sulphate .
Aims
Soluble aluminium often abbreviated to Al3+ is toxic to plants. The occurrence of this species of Al in the soil layers below 10 cm (subsoil) is a major problem in the south Western Australian agricultural region due to acidic soil, particularly in the subsurface and subsoil (Gazey et al. 2014). As the soil solution pH (pHSoln) declines below 5.0 Al3+ and other species of Al concentrations in the soil solution increase (Rutkowska et al. 2015). That is hydrolysis of Al3+ breaks the H-O bond and the release of hydrogen ions results in the formation of; , , . Also Al3+ can form complexes with fluorine (F), phosphate (P), sulphate (S) and organic matter (OM) which are not toxic to plants. In general the order of decreasing toxicity of Al species is; , > > > > inert and (Rutkowska et al. 2015).
Lime (CaCO3) and gypsum (CaSO4) can be used to treat the soil which has toxic levels Al3+ increasing wheat grain yield (McLay et al. 1994; Easton 2016). The application of lime has the effect of increasing soil pH as measured using 0.01 M CaCl2 extracting solution (pHCa) resulting in the conversion of Al3+ to inert gibbsite (Rutkowska et al. 2015). While the sulphate component of gypsum is leached into the subsoil resulting in toxic Al species such as Al3+ been changed to inert aluminium sulphate () and . There has been a large increase in lime use in the south Western Australia agricultural region over the period 2004-2013 to manage soil acidity (Gazey et al. 2014). However, gypsum is rarely used to treat subsoil Al toxicity in the wheat belt of WA. In contrast, gypsum is commonly used in Brazil for treating subsoil Al toxicity (Tiecher et al. 2018).
This paper aimed to determine whether the concentration of Al in soil solution (AlSoln) and the species of Al had changed following the application of lime and gypsum to an acidic soil near Bonnie Rock. It was hypothesised that changes in Al concentration and species could explain the increase in wheat grain yield observed when lime and gypsum had been applied (Easton 2016).
Method
An experiment examining the effect of lime and gypsum located near Bonnie Rock (370 km north east of Perth) was conducted over the period 2008-2016 (Easton 2016). The experiment used limesand (effective neutralising value of 80%), dolomite (effective neutralising value 90%) and an industrial gypsum source (Ca content of 22.4% and an S content of 17.8%). Treatments were applied in 2008 and again in 2013, although with dolomite sourced from Beaufort River (effective neutralising value 60%). This gives eight treatments; control, 2, 4 and 8 t/ha limesand, 2 t/ha gypsum, 4 t/ha lime + 2 t/ha gypsum, 4 t/ha dolomite, 4 t/ha dolomite + 2 t/ha gypsum replicated three times (Easton 2016).
On 21 March 2018 soil samples were collected only from the lime by gypsum factorial combination control, 4 t lime/ha (lime), 2 t gypsum/ha (gypsum) and 4 t lime/ha plus 2 t gypsum/ha (lime plus gypsum) treatments of the experiment. Four soil cores were collected from each plot and divided into increments of 10 cm to a depth of 50 cm. The layer samples of the four soil cores were then bulked together to provide a measurement of the individual treatment plot of each replicate. Soil chemical properties and gravel content were determined using standard soil analytical techniques for the control treatment (Table 1).
Table 1. Summary of measured chemical properties and gravel content of the control treatment measured on 21 March 2018.
Depth | Carbon | Gravel | Colwell P | PBI | KCl 40 S | SBIA | pHCaCl2 | AlCaCl2 | Alex |
|---|---|---|---|---|---|---|---|---|---|
Cm | (%) | (%) | (mg/kg) | (mg/kg) | (mg/kg) | (%) | |||
0-10 | 0.8 | 9% | 25 | 31 | 15.5 | 13 | 4.6 | 3.1 | 15 |
10-20 | 0.5 | 16% | 9 | 40 | 15.9 | 10 | 4.5 | 11.4 | 42 |
20-30 | 0.4 | 55% | 3 | 40 | 23.6 | 14 | 4.5 | 11.1 | 42 |
30-40 | 0.3 | 55% | 3 | na | 22.8 | 12 | 4.5 | 6.9 | 31 |
40-50 | 0.4 | 58% | 2 | na | 28.4 | 14 | 4.7 | 6.0 | 27 |
ASBI refers sulphate buffer index which is a modification of the procedure for measuring the S adsorption presented by Anderson and Fillery (2006).
The soil solutions were extracted using Milli Q water at the soil to solution ratios (S/L ratio) of 1/0.4 or 0.4 ml/g for the 0–10 and 10–20 cm soil layers and 1/0.35 or 0.35 ml/g soil for the other three depths. The different ratios were due to the different moisture retention at these depths. These S/L ratios were used to enable the collection of sufficient soil solution to undertake the measurements. An amount of 200 g of soil was added to a 500 mL beaker. The amount of Milli Q water depending upon the moisture retention of soil was added to the soil, covered with glad wrap to reduce the evaporation loss of water and incubated for 16 hours. The soil solution was then extracted by centrifuging the soil for 10 minutes at 10,000 rpm to extract the soil solution. An aliquot sample was passed through a 0.45 µm screen cellulose membrane.
Soil solution was analysed for alkalinity or the amount of acidity required to lower soil solution pHSoln to 4.3 (mg/L), CaSoln (mg/L), FeSoln (mg/L), KSoln (mg/L), Mg (mg/L), NaSoln (mg/L), PSoln (mg/L), S (mg/L), SiSoln (mg/L) were measured using an ICP–AES. Redox potential or EhSoln (mV), pHSoln and FSoln (mg/L) were measured using an ion specific electrode. Nitrogen concentration as NH3 (mg/L), NO2 (mg/L) and NO3 (mg/L) was measured calorimetrically. Dissolved organic carbon (DOCSoln) (mg/L) was determined using a modified Walkley and Black (1934) method for soil solution using a sample size of 5 ml. The measured nutrient concentrations were converted to concentration in the soil solution with a water content of 14% for the 0–10 cm soil layer and 10% for the soil layers below 10 cm. These values were then entered into the chemical equilibrium model PHREEQC (Parkhurst and Appelo 1999) to estimate the composition of chemical species. Individual replicate samples were used to extract soil solution but only treatment average concentrations have been used in PHREEQC.
Analysis of variance using Genstat® was conducted on the soil solution chemical properties of the soil profile to a sampling depth of 50 cm. The analysis of variance used a treatment structure of treatment and soil sampling depth and a block structure of replicate to determine the significance of treatment, soil depth, and treatment x depth interaction. In the analysis of variance soil depth was used as a covariate. Differences between treatments, soil sampling depth and treatment x sampling depth interaction were determined using l.s.d. values at P = 0.05.
Results
Note that all of the results reported below relate to soil samples collected in 2018, that is ten and five years respectively after the original and follow-up treatments were applied.
Soil solution concentrations
The pHSoln for the lime treatment was higher than the control treatment in the 0–10 cm soil layer (6.9 versus 4.6) and in the 10–20 cm soil layer (5.4 versus 4.3) (Figure 1a). In contrast, gypsum application did not affect pHSoln. A comparison between soil and solution pH measurements illustrate, soil pHCa was strongly related to the pHSoln (pHsoln= 1.45 x pHCa – 1.91, r2 = 0.91) with the difference between pHCa and pHSoln increasing with increasing pHCa.
The AlSoln concentration for the lime treatment was lower than the control treatment in the 0–10 cm soil layer (0.1 mg Al/L versus 1.0 mg Al/L) and in the 10–20 cm soil layer (0.5 mg Al/L versus 1.7 mg Al/L) (Figure 1b). In contrast, AlSoln concentration for the gypsum treatment was 2.3 mg Al/L in the 0–10 cm soil layer, 3.2 mg Al/L in the 10–20 cm soil layer, 2.0 mg Al/L in the 20–30 cm soil layer and 1.1 mg Al/L in the 30–40 cm soil layer. While AlSoln concentration for the lime plus gypsum treatment was 0.0 mg Al/L in the 0–10 cm soil layer, 0.4 mg Al/L in the 10–20 cm soil layer, 0.6 mg Al/L in the 20–30 cm soil layer and 0.5 mg Al/L in the 30–40 cm soil layer.
The SSoln concentration for the gypsum treatment was higher than the control treatment in the 0–10 cm soil layer (1426 versus 182 mg S/L), in the 10–20 cm soil layer (285 versus 80 mg S/L), in the 20–30 cm soil layer (214 versus 62 mg S/L) and in the 30–40 cm soil layer (200 versus 53 mg S/L) (Figure 1c). In contrast, SSoln for the lime plus gypsum treatment was 639 mg S/L in the 0–10 cm soil layer and between 317-585 mg S/L in the 10–40 cm soil layer.
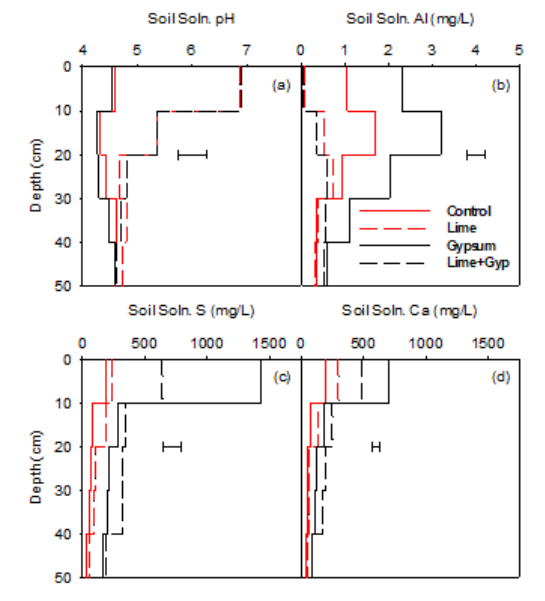
Figure 1. Impact of lime and gypsum treatments on soil solution (a) pHSoln, (b) AlSoln mg Al/L (c) SSoln mg S/L and (d) CaSoln (mg Ca/L) measured on 21 March 2018.
The CaSoln concentration for the lime plus gypsum treatment was higher than the control treatment in the 0–10 cm soil layer (481 versus 197 mg Ca/L), in the 10–20 cm soil layer (250 versus 71 mg Ca/L), in the 20–30 cm soil layer (192 versus 50 mg Ca/L) and in the 30–40 cm soil layer (175 versus 47 mg Ca/L) (Figure 1d). CaSoln for the gypsum treatment was the highest, with 700 mg Ca/L in the 0–10 cm layer, 115 mg Ca/L in the soil layer 10–20 cm layer and 75 mg Ca/L in the soil layer 20–30 cm layer. Application of lime resulted in a CaSoln concentration of CaSoln 298 mg Ca/L in the 0–10 cm layer and 139 mg Ca/L in the 10–20 cm layer
Modelled Al species within the soil solution.
The main modelled AlSoln species present for the control treatment were Al3+, and which was predicted to account for 77–96% of AlSoln (Figure 2). Al3+ was predicted to account for 38% of AlSoln in the 0–10 cm soil layer increasing to 63% in the 40–50 cm soil layer. The was predicted to account for 20–23% of AlSoln in the 0–20 cm soil layer and 4–11% in the 20–50 cm soil layer. AlSoln as and declined from 32% in the 0-10 cm soil layer to 15% in the 40-50 cm soil layer.
Gypsum application had a low percentage of AlSoln as Al3+, 18% in the 0–10 cm and to 37% in the 40–50 cm. In contrast, the percentage of AlSoln as and was high (53-67%) in the soil profile. While the percentage of AlSoln as was stable in the 0–40 cm soil layer (6–22%) with only 6% present in the 40–50 cm soil layer.
Lime application had low percentage of AlSoln as Al3+ (0-3%) and a high the percentage of AlSoln as in the 0-20 cm soil layer (87%) and in the 20-50 cm soil layer (40-60%). The next most common Al species was accounting for 7% of AlSoln in the 0–20 cm soil layers increasing to 18–26% in the 20–50 cm soil layers. There were low amounts of and accounting for 0-4% of AlSoln and accounted for 6–15% of AlSoln in the 0–50 cm soil layers.
Lime plus gypsum application also had a low the percentage of AlSoln as Al3+ to 0-1% in the soil profile and high levels of in the 0-20 soil layer (91%). There was a high percentage of AlSoln as (42-60%) and (16-22%) in the 10-50 cm soil layers. There were also low amounts of and accounting for only 4-10% of the AlSoln.
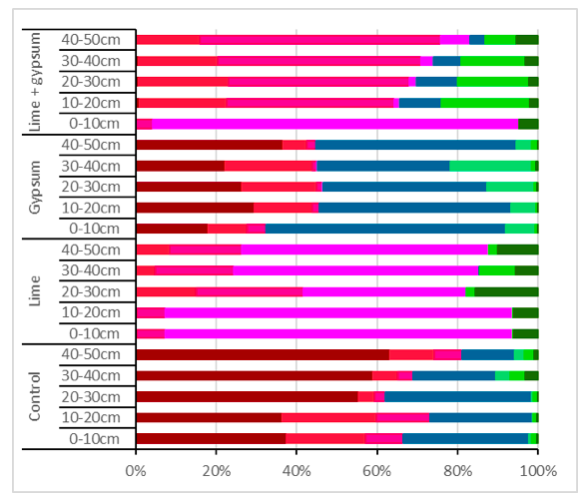
Figure 2. Impact of lime and gypsum treatments on Al species present in the soil solution ten years after initial application. Al species are presented in order of decreasing toxicity with Al3+ (■), > (■), > (■), > (■), (■), (■), (■) and (■) in the soil layers to a depth of 50 cm calculated using PHREEQC. The red shaded species indicate the forms toxic to plant while the green shaded species are not toxic.
Conclusion
Lime and gypsum application changed the AlSoln concentration (Figure 1b) and Al species distribution in the soil solution (Figure 2). The lime application increased pHSoln and CaSoln concentration and decreased AlSoln (Figure 1) resulting in the conversion of Al3+ to the less toxic species , and (Figure 2). The effect of lime application on pHSoln and AlSoln was measured to a depth of 20 cm (Figure 1) while the effect on Al species distribution occurred to a depth of 50 cm (Figure 2). Indicating assessing the Al species distribution is a more sensitive measure of the impact of lime than either AlSoln or pHSoln. In contrast, gypsum did not affect pHSoln but the increased SSoln and AlSoln concentration (Figure 1). The increase in SSoln occurred to a depth of 50 cm. An increase SSoln results in toxic Al3+ being predicted to be converted to inert (Figure 2, Sumner 1990). However, because gypsum application has increased AlSoln concentration (Figure 1b), a lime application was also required to remove Al3+ from the soil profile (Figure 2). These changes in AlSoln species distribution likely contributed to the increase in wheat grain yield over the control of 4-32% when gypsum was applied, of 3-33% when lime was applied and of 17-48% when both lime plus gypsum was applied (Easton 2016).
The ability of S to reduce Al toxicity in soils depends on the SSoln concentration achieved by the gypsum application (Cameron et al. 1986). The S adsorption properties of the soil control the SSoln concentration with soils having higher S adsorption capacity increases the amount of gypsum requirement to treat subsoil Al3+ (Sumner 1990). The soil profile in this experiment had a relatively low capacity to adsorb S as indicated by the low SBI values of the soil profile (Table 1). The low S sorption ability means the soil had a relatively high SSoln concentration (Figure 1c) for the measured content of SSoil. This interaction between SSoil, SSoln and percentage of SSoil as is illustrated by the changes in these soil properties with increasing soil depth for the control treatment. As soil depth increases, both SSoln concentration and the percentage of AlSoln as was was predicted to decrease while SSoil was observed to increase. Hence, the sulphate adsorption capacity of the soil increased with soil depth resulting in a decrease in the percentage of AlSoln as . The S content of the soil (SSoil) as measured by the KCl-40 extraction technique of the 0-30 cm soil layer (Table 1) was much greater than the critical value of 2.8 mg S/kg required for crop nutrition (Anderson et al. 2013). Therefore, crop responses to gypsum application were highly unlikely to be associated with improved S nutrition.
Gypsum application increased the concentration of CaSoln (Figure 1d) which would result in the displacement of Al3+ from the exchange sites (Mora et al. 1999) and an increase in the concentration AlSoln (Figure 1b,). Nevertheless, for the gypsum treatment was predicted to have lower Al3+ than the control treatment due to the formation of (Figure 2).
Gypsum with S content of 17.8% applied at 2t/ha was observed to be effective in treating subsoil Al toxicity on a soil profile with relatively low sulphate adsorption capacity as indicated by the SBI measurement. The low SBI values of the soil indicate gypsum-S would have been leached rapidly into the soil profile to produce a wheat grain yield response in the first year of application (Easton 2016). But leaching of SSoln over time was observed to reduce the effectiveness of the applied gypsum. Further research should be conducted to examine the effectiveness of gypsum in treating subsoil Al toxicity over time and on soils with higher SBI.
Further information Geoff Anderson 08 96902104 or geoff.anderson@dpird.wa.gov.au
Acknowledgements
GRDC project number DAW00242
Reviewed by Dr James Fisher
References
Anderson G, Peverill K, Brennan R (2013) Soil sulfur – crop response calibration relationships and criteria for field crops grown in Australia. Crop and Pasture Sciences 64, 523–530
Anderson GC, Fillery IRP (2006) Sulfate sorption by agricultural soils in Western Australia. World Soil Science Congress, Philadelphia, USA, 9th – 15th July 2006.
Cameron RC, Ritchie GSP, Robson AD (1986) Relative toxicities of inorganic aluminium complexes to barley. Soil Science Society America Journal 50, 1231-1236.
Easton J (2016) Soil Acidity – Can Gypsum Increase Wheat Yields on Aluminium Toxic Soils? In 2016 GRDC Research Updates 29-30 February 2016 .
Gazey C, Davies S, Master R (2014) Soil acidity – A guide for WA farmers and consultants, Second edition. Department of Agriculture and Food, Western Australia, Bulletin 4784. ISSN 1833-7236
McLay CDA, Ritchie GSP, Porter WM, Cruse A (1994) Amelioration of subsurface acidity in sandy soils in low rainfall regions. I. Responses of wheat and lupins to surface-applied gypsum and lime. Australian Journal of Soil Research 32, 835–846
Mora ML, Schnettler B, Demanet R (1999) Effect of liming and gypsum on soil chemistry, yield, and mineral composition of ryegrass grown in an acidic Andisol. Communications in Soil Science and Plant Analysis 30, 9-10.
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2) A computer program for speciation, batch–reaction, one dimensional transport, and inverse geochemical calculations: U.S. Geological Survey Water–Resources Investigations Report 99–4259, 0-310.
Rutkowska B, Szulc W, Hoch M, Spychaj–Fabisiak E (2015) Forms of Al in soil and soil solution in a long–term fertilizer application experiment. Soil Use and Management 31, 114–120.
Sumner ME (1990) Gypsum as an ameliorant for the subsoil acidity syndrome. Publication No 01–024–090. Florida Institute of Phosphate Research, Bartow.
Tiecher T, Pias OHC, Bayer C, Martins AP, Denardin LGO, Anghinoni I (2018) Crop response to gypsum application to subtropical soils under no-till in Brazil: a systematic review. Rev Bras Cienc Solo. 42:e0170025.
GRDC Project Code: DAW00242,
Was this page helpful?
YOUR FEEDBACK
