Sustaining our herbicides into the future
Author: Christopher Preston, Peter Boutsalis, Patricia Adu-Yeboah and Gurjeet Gill (The University of Adelaide) | Date: 31 Jul 2019
Take home messages
- Resistance to post-emergent herbicides is present in annual ryegrass, brome grass and barley grass populations on the northern Eyre Peninsula.
- Control of barley grass with pre-emergent herbicides is more difficult in low rainfall zones due to the low solubility of the herbicides and the patchy nature of rainfall events.
- Getting better control of barley grass in the pasture phase is the key to reducing populations in the cereal phase.
Herbicide resistance on the Eyre Peninsula
The last survey for herbicide resistant weeds on the Eyre Peninsula was in late 2014. Annual ryegrass was the most common weed found in 85% of crop fields surveyed. In the northern Eyre Peninsula, there was a high frequency of resistance to the Group B herbicides, including Intervix® (Table 1). Resistance to trifluralin and Group A fop herbicides was at 10% and no resistance was identified to the other herbicides. There was considerably more resistance identified in the southern Eyre Peninsula, consistent with annual ryegrass being a greater problem and continuous cropping being more common in the south.
Table 1. Annual ryegrass populations resistant to herbicides for the Eyre Peninsula survey in 2014. Populations are considered resistant if 20% of individuals survived the herbicide.
Herbicide | Southern | Northern |
|---|---|---|
Populations resistant (% tested) | ||
Trifluralin | 51 | 10 |
Propyzamide | 0 | 0 |
Sakura® | 0 | 0 |
Avadex®Xtra | 3 | 0 |
Boxer Gold® | 1 | 0 |
Diclofop-methyl | 73 | 10 |
Axial® | 32 | 0 |
Clethodim | 7 | 0 |
Chlorsulfuron | 85 | 75 |
Intervix® | 53 | 39 |
Glyphosate | 1 | 0 |
Brome grass was collected from 28% of paddocks visited and was concentrated in the northern Eyre Peninsula. Of these populations, 52% were resistant to Atlantis® (Group B) and 4% resistant to Targa® (Group A) (Table 2). No resistance was identified in the survey to either Intervix® or glyphosate. Both B. diandrus and B. rigidus were present among resistant populations.
Table 2. Brome grass, barley grass and wild oats populations resistant to herbicides for the Eyre Peninsula survey in 2014. Populations are considered resistant if 20% of individuals survived the herbicide.
Herbicide | Brome grass | Barley grass | Wild oats |
|---|---|---|---|
Populations resistant (% tested) | |||
Targa® | 4 | 4 | - |
Topik® | - | - | 4 |
Atlantis® | 52 | 16 | 3 |
Intervix® | 0 | 0 | - |
Glyphosate | 0 | - | - |
Barley grass was collected from 37% of paddocks visited, confirming the increasing importance of barley grass as a weed of cropping on the Eyre Peninsula. For barley grass there was a low frequency of resistance to Group A herbicides, but 16% of populations were resistant to Group B herbicides. Both H. leporinum and H. glaucum were represented in resistant populations.
Wild oats was collected from 18% of paddocks visited, primarily in the southern Eyre Peninsula, with both A. sterilis and A. fatua present. A small number of populations had resistance to Topik® and to Atlantis® (Table 2).
Two broadleaf weeds were collected during the Eyre Peninsula survey: Indian hedge mustard and sowthistle. These species were present in 18 and 11% of paddocks surveyed, respectively, and so are much less common than grass weeds. High frequencies of resistance to the Group B herbicides were present in both species (Table 3). Indian hedge mustard populations were also resistant to diflufenican, atrazine and 2,4-D.
Table 3. Indian hedge mustard and sowthistle populations resistant to herbicides for the Eyre Peninsula survey in 2014. Populations are considered resistant if 20% of individuals survived the herbicide.
Herbicide | Indian hedge mustard | Sowthistle |
|---|---|---|
Populations resistant (% tested) | ||
Chlorsulfuron | 64 | 75 |
Eclipse® | 74 | - |
Intervix® | 14 | - |
Diflufenican | 36 | - |
2,4-D | 7 | 0 |
Atrazine | 7 | 0 |
Glyphosate | 0 | 0 |
The challenges of pre-emergent herbicides in low rainfall environments
With the evolution of resistance to post-emergent herbicides in weeds like barley grass, inevitably there will be more reliance on pre-emergent herbicides to control these weed species. There are now a range of pre-emergent herbicides that can be used in various phases of the rotation for the control of grass weeds. Barley grass is listed as a target weed on many of the product labels; however, it is apparent that several of these herbicides can provide variable performance in low rainfall zones.
The main reason for this poor performance is that many pre-emergent herbicides have limited water solubility. This is often a useful trait in pre-emergent herbicides, as it means they do not move too far in the soil profile. However, it presents challenges in getting good activity in low rainfall environments. In order to better understand the performance of pre-emergent herbicides in low rainfall environments an experiment was conducted where a range of pre-emergent herbicides were applied on top of barley grass seed buried five mm in soil. The herbicides were then incorporated by another 1 cm of soil. After herbicide application, half of the pots were watered as normal and the other half were protected from rainfall for seven days and then watered as normal.
This procedure mimicked a scenario where rain had occurred prior to sowing the crop, but there was no further rainfall for a week after sowing. Trifluralin provided relatively little control of barley grass under either situation (Figure 1) but was slightly more effective under dry conditions than wet conditions. F9600, a new active ingredient likely to be registered by 2021, was also not very effective on barley grass in this trial. Propyzamide, Sakura® and Ultro reduced barley grass emergence under the wet conditions. Under dry conditions, there was a significant reduction in control of barley grass with Sakura® and propyzamide compared to wet conditions, but not for Ultro (Figure 1). This is in keeping with the inconsistent performance of Sakura® against barley grass in low rainfall environments.
n.b Ultro (carbetamide, Group E) is currently in development and an application for registration has been submitted to the APVMA. Growers must not use until notification of registration
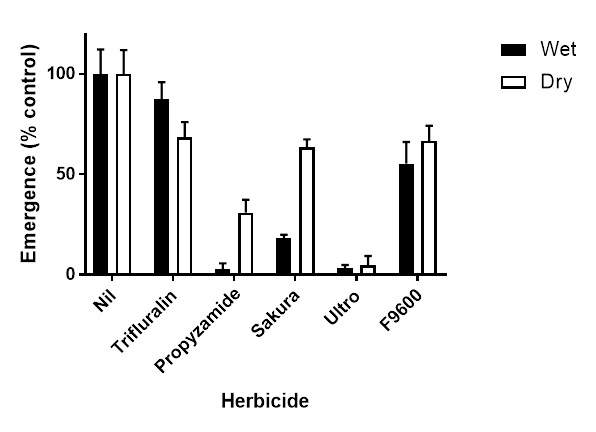
Figure 1. Effect of various pre-emergent herbicides on the emergence of barley grass under wet or dry situations at crop seeding.
Propyzamide and pyroxasulfone (the active ingredient in Sakura®) have relatively low water solubility. This explains their lower efficacy in low rainfall situations. Carbetamide (the active ingredient in Ultro) has much greater water solubility. When using lower solubility pre-emergent herbicides in low rainfall environments it is important to have some rainfall within a few days after application to get the best efficacy out of the products.
Barley grass and brome grass have both evolved resistance to glyphosate
In the past 10 years, glyphosate resistance has been identified in both brome grass and barley grass. Resistance has occurred on fence lines, old fence lines or similar situations. The ability of barley grass seed in particular, to move through the environment, means that resistance can easily spread. Glyphosate resistant barley grass was identified in 2017 on the Yorke Peninsula in South Australia. The two resistant populations, YP1 and YP2, require three to six times as much glyphosate for control compared to normal susceptible populations (Figure 2).
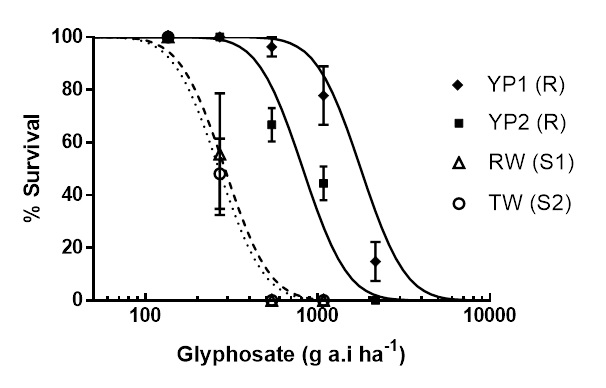
Figure 2. Response of glyphosate-resistant barley grass populations YP1 (♦) and YP2 (■) and susceptible populations RW (Δ) and TW (○) to glyphosate.
Integrated management of barley grass and brome grass
Barley grass and brome grass can be difficult to manage because there are relatively few herbicides available to control them in cereals, except in ClearfieldTM crops. In both species, there has been selection for greater dormancy in cropping systems, so the weeds are avoiding knockdown herbicide applications. They also readily persist in pasture systems. The seeds shatter prior to harvest, making harvest weed seed control practices less effective.
Previous work with brome grass management demonstrated two years of good control could run weed seed banks down to low levels. This could be achieved through double break crops or a break crop followed by a ClearfieldTM cereal. In the break crop it was important to stop brome seed set and in the cereal crop, use of crop competition as well as Intervix® was important.
Barley grass has a relatively short seed bank life and it should be easier to reduce weed populations than for brome grass. This is certainly true where pulse crops are part of the rotation, because there is the opportunity to stop seed production in pulses. However, cereal and pasture rotations are leading to increases in barley grass populations. Barley grass is often seen as providing valuable early feed in pastures and is therefore tolerated early. Stopping seed set of barley grass in pastures is often difficult. The main practice used is pasture topping, but this relies on being able to graze hard, take the stock off and apply paraquat once the barley grass starts to flower. Most often, pasture topping is not conducted at the appropriate time, so some barley grass seed sneaks through control efforts. Rainfall after the herbicide application can also cause problems, as barley grass can send up new seed heads and a second pasture topping application may be required.
Reducing the barley grass population size earlier in the pasture prior to pasture topping will make it easier to get a good result with pasture topping, as the flowering of barley grass will become more compressed. Using a double knock approach, where glyphosate is applied earlier at flowering and paraquat is used to control regrowth, will also provide better results.
Useful resources
Alternative Herbicides for Management of Group A Herbicide-Resistant Barley Grass in Field Pea
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support.
Contact details
Christopher Preston
University of Adelaide
0488 404 120
christopher.preston@adelaide.edu.au
Was this page helpful?
YOUR FEEDBACK
