Doing invertebrate pest management better
Author: Melina Miles (Queensland Department of Agriculture and Fisheries). | Date: 18 Feb 2020
Take home messages
- Grower engagement is essential to achieving changes in practice with pest management.
- Think small when considering changes. Set specific goals, plan small steps and review what happened.
- Better pest management outcomes are achieved through incremental improvements in the basics: knowledge of key pests, planning management strategies, identification, sampling, use of thresholds and/or risk assessment.
Background
Currently in the grains industry, particularly in the winter cropping dominated regions, the management of invertebrate pests is dependent on the use of insecticides. Insecticides are extremely useful options in pest management, but frequently, applications of insecticides in the grains industry are prophylactic. In other words, they are applied before pest populations eventuate, or reach a recommended threshold. These applications are typically via seed treatments, a bit of insecticide in the tank with something else (just in case), and/or to supress a possible outbreak between farm visits. What this means is that a lot of insecticide goes on ‘just in case’. It is costly and has consequences for the development of insecticide resistance and the loss of ‘free’ pest control by also killing off natural enemies (beneficials).
During 2013 to 2015, entomologists (DAF, cesar, SARDI) held 24 workshops across the southern and northern grain regions to discuss pest management and the aspirations of agronomists and growers. Key to the conversation was an understanding of why insecticides are so dominant in invertebrate pest management. Agronomists identified the following as major contributing factors:
- Low cost of insecticides.
- Growers not engaged in invertebrate pest management; leaving it to the agronomist who may not be prepared to take on the risk of doing things differently.
- Invertebrate pest management is reactive; not discussed when planning.
- Time constraints: common perception that the time required to check crops and plan/implement non-chemical management strategies is too costly in comparison with simply applying insecticides. The current agronomist:client ratio in most regions contributes significantly to this.
- Low confidence in available thresholds to guide decision making.
- A low or zero tolerance for damage that is visible to the grower in the field, or at delivery. For example; faba bean standards, white pods visible in chickpea paddocks, etc.
Despite this long list of reasons to continue doing what you are doing, there is an increasing number of growers and agronomists interested in how they might do things differently. There are also many growers and agronomists in the northern region (particularly north of Dubbo) who routinely plan their pest management strategy ahead of time, regularly check crops, record pest and beneficial numbers, use economic thresholds to guide decisions and apply selective insecticides where appropriate.
The aim of this paper (and presentation) is to provide a few examples of ‘small steps’ that could improve your pest management approach and efficacy.
Discussion
Planning to manage, not just control
Example: Establishment pests in canola
Fundamental to reducing reliance on insecticides is knowing when a crop is at risk of loss from pests and when it isn’t. Knowing which environmental conditions promote or suppress specific pests is vital in planning. In high risk situations, application of insecticides to prevent irrecoverable crop loss is essential. Where risk is lower, it is possible to monitor and treat crops only as necessary. Table 1 is an extract of a more detailed table (which can be found at: ‘Best Bet’ IPM strategy Establishment pests – Southern region that describes the conditions that contribute to high and low risk of damaging earth mite and lucerne flea infestations. It is a useful starting point for discussion about the level of risk you are facing and how it might be mitigated.
When planning for the season, you identify and discuss relevant factors. For example; crop rotations, weed management, seasonal conditions/forecasts and expectations of the coming season (amongst other things). While planning, it is also the time to discuss what insecticide modes of action (MOA) are available and how they will be deployed given different scenarios of pest outbreak. This way the grower and agronomist are in agreement on how to proceed during the season, reducing the reactiveness of decisions.
In this example, the small step towards change is: Grower and agronomist discuss likely establishment risks to one canola crop and an approach to pest management that is tailored to that paddock. Document, implement, review.
Table 1. Best bet insect pest management (IPM) strategy for establishment pests (southern region). Extract of table that can be found at: ‘Best Bet’ IPM strategy Establishment pests – Southern region
Pre-season | Pre-sowing | Emergence | Crop establishment | |
|---|---|---|---|---|
Earth mites & lucerne flea | Assess risk. High risk when: · history of high mite pressure · pasture rotating into crop · susceptible crop being planted (e.g. canola, pasture, lucerne) · seasonal forecast is for dry or cool, wet conditions that slow crop growth. If risk is high: · ensure accurate identification · use Timerite (redlegged earth mites only) · heavily graze pastures in early‐mid spring | If high risk: · use an insecticide seed dressing on susceptible crops · plan to monitor more frequently until crop establishment · use higher sowing rate to compensate for seedling loss · consider scheduling a post‐emergent insecticide treatment If low risk: · avoid insecticide seed dressings (esp. cereal and pulse crops) and plan to monitor until crop establishment | Monitor susceptible crops through to establishment using direct visual searches. Be aware of edge effects; mites move in from weeds around paddock edges If spraying: · ensure accurate identification of species before deciding on chemical · consider border sprays (mites) and ‘spot’ sprays (lucerne flea) · spray prior to winter egg production to suppress populations and reduce risk in the following season | As the crop grows, it becomes less susceptible unless growth is slowed by dry or cool, wet conditions |
Using thresholds and experience to guide decisions
Without a sound grasp of whether or not a crop is infested with a potentially damaging pest population, agronomists and growers frequently resort to spraying. Where thresholds are available, they provide guidance as to what density of pests will cause economically significant crop loss. Below this level you may see damage in the crop, but it will either not result in crop loss (yield or quality), or the value of the loss will be less than the cost of spraying. Where there are no thresholds, experience is a good guide as to whether treatment is required. However, if the default response to finding pests in a crop is to spray, this may indicate that the agronomist/grower may not have the experience in determining how much damage a pest infestation will cause, and if not sure and can’t take the risk; inevitably will spray. If you spray because of uncertainty, a small step in changing practice is to leave a small unsprayed area in which to learn from. For example, turn the boom off for the last 50m of the last run. Monitor this area regularly during the season recording the density and size of pests and the number of natural enemies. Compare what happens here with the rest of the field (for example, the number of damaged and undamaged pods/heads per metre of row). If possible, make the untreated area big enough to assess with the yield monitor at harvest. Review the outcome to determine if your sprayed area performed better than the unsprayed areaa. Don’t change what you do in every paddock as a result of this one experiment but use it to build your experience in pest management. Repeat next year with a different crop or pest and share the result with your client/agronomist.
Sampling with confidence
Example: Helicoverpa in faba beans, armyworm in winter cereals
Sampling is fundamental in order to make decisions about management and/or control. Knowing a little about the behaviour of the pest in the crop and the effectiveness of available sampling methods will help identify where a different method, or combination of methods will give the best information.
In the examples to follow, the sampling data includes both an estimate of density and the size of larvae as the most useful. For both helicoverpa and armyworm, the treatment with greatest efficacy is achieved (and risk reduced) when small larvae are targeted, before they start to damage grain.
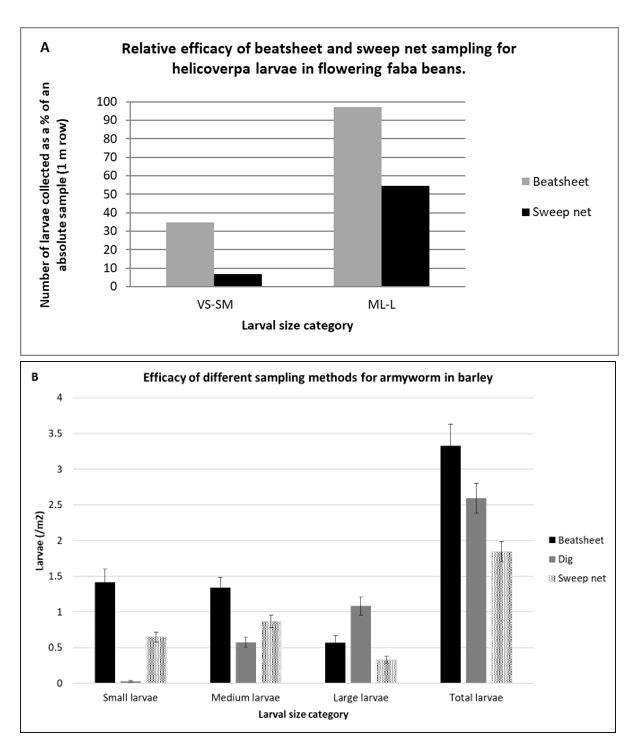
Figure 1A shows how the sampling method used to assess helicoverpa larvae in flowering faba beans can give very different results. The discrepancies are a result of both larval behaviour and the penetration of the canopy by each sampling method. Larger larvae (ML-L) are easier to dislodge from the crop than are the smaller larvae, and the beatsheet does this much more effectively than the sweep net. The likely explanation is that the beatsheet can dislodge larvae from the full height of the canopy, the sweep net only from the top. When it comes to estimating the density of the smaller larvae (VS-SM) neither method does this well enough for good decision making. As a result of having this data, the recommendation for sampling helicoverpa in faba beans in the northern region now suggests that the agronomists make a visual inspection of flowers and terminals, looking specifically for small larvae, in addition to beatsheet sampling.
Figure 1B shows how essential it is to include digging along the row when sampling for armyworm, regardless of whether you use a beatsheet or sweep net. Otherwise, the number of larger, damaging large larvae is severely underestimated.
Figure 1. (A) Relative efficacy of beatsheet and sweep net sampling methods for detecting helicoverpa larvae in flowering faba beans. Relativity established through absolute sampling (looking at what was left). Larval size categories are: VS-SM=very small to small medium (1st-3rd instar), ML-L=medium to medium large (4th – 6th instar).
(B) Distribution of larvae in the canopy of flowering faba beans as a percentage of total larvae.
Harnessing the contribution of beneficials
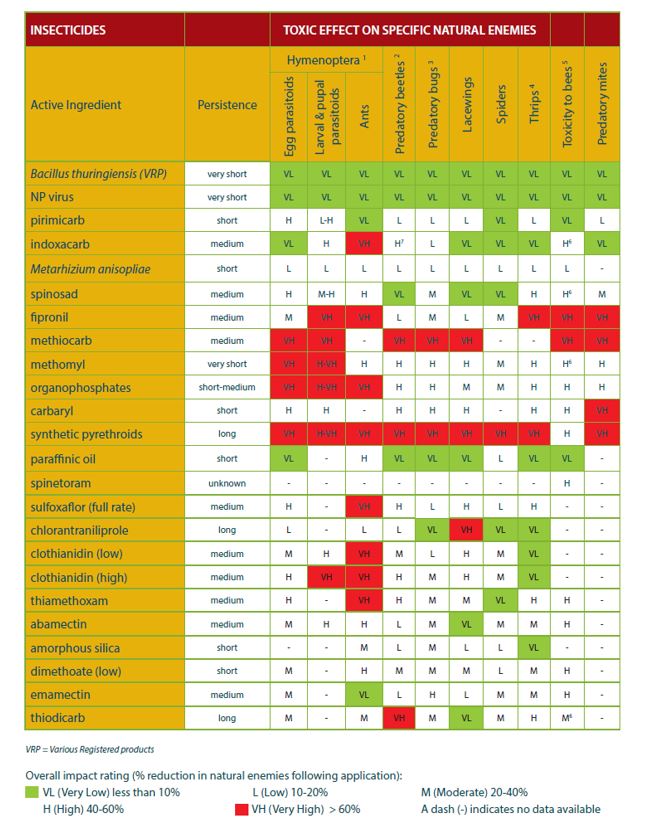
Beneficials, in a broadacre context, include both natural enemies (predators, parasitoids, diseases), pollinators and decomposers in the soil. Natural enemies play an important role in suppressing pest populations in-crop and in fallows. The use of broadspectrum insecticides, and particularly the repeated use of these in a crop and across seasons, reduces the potential contribution that beneficials can make. The use of broadspectrum insecticides to control helicoverpa, Etiella, diamondback moth and/or aphids will impact on pollinators (bees, wasps, flies) in pulses and canola, as well as on the natural enemies. A small step worth taking is to review what products, and how many applications, for your canola crop in a season. Figure 2 ranks registered insecticides from low to high in terms of their detrimental impact on natural enemies. These data are largely based on one exposure to one active, and therefore, consider how much impact multiple applications are having, and whether there are opportunities to reduce it.
Figure 2. Impact of insecticides on natural enemies in crop (Source: Extracted from I-Spy – Insects of the Southern Australian Broadacre Farming Systems Identification manual and educational resource (2nd edition)).
Food for thought - options for the future
Selective insecticides
Selective insecticides that have reduced off-target impacts are widely used in the northern region for control of caterpillar pests (for example; Altacor®, Steward®, Affirm®, Success Neo®) and aphids (for example; pirimicarb). In the case of helicoverpa control, they are used principally because H. armigera is dominant and resistant to many older products. In addition to being less disruptive to beneficials, these products typically have longer residual efficacy than synthetic pyrethroids or organophosphates. It is a common perception that the higher cost of these products can’t be justified, however deployed strategically they offer benefits. In the southern region, the development and spread of insecticide resistance in green peach aphid and redlegged earth mite will make the use of alternate products essential.
Models to assist with decision making
Models that predict the rate of pest development will be useful to bridge the uncertainty about what may happen in the paddock between the checks. Networks of traps for key species (helicoverpa, armyworm, aphids) could generate additional early warning of pest outbreaks. Greater certainty will potentially reduce the number of prophylactic treatments. SARDI has developed a model to predict the timing of Etiella flights into lentils, allowing for better targeting of egg-laying moths. Similar models have been developed for Rutherglen bug and barley yellow dwarf virus (BYDV)-carrying aphids but are not widely deployed.
Tweaking the system to disadvantage the pests/advantage the crop
Where pest risk is persistently high, changes to the system may provide reprieve, although it is not necessarily a straightforward proposition. For example, in some regions of Western Australia (WA), canola planting has shifted earlier to take advantage of the crop growth benefits of warmer conditions at emergence. Unexpectedly, the warmer conditions at emergence and establishment are also suitable for a suite of invertebrate pests that would normally be absent or less active under cooler conditions, increasing the pest pressure and consequently the number of sprays applied.
Conclusion
There are a multitude of reasons that growers and agronomists decide they need to change what they are doing when it comes to invertebrate pest management. It may be that traditional practices are not working as well as they did, they just don’t want to spray as often as they do, or they have seen that increased diversity in their landscape results in lower pest pressure. Once you look, you will find that there are many tools, options and information to assist you in making changes.
Whatever the motivation, identifying where to start can be daunting. ‘Think small’ is a useful way to approach this challenge. Identify just one pest, one thing you want to achieve. Make a plan, put it into action and monitor and record what happens. Share what you are doing and finding and repeat.
Acknowledgements
The research undertaken as part of these projects (DAQ00196, DAQ00179) is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them, and their agronomists, for their continued support. The trial results presented were possible through the technical support of Adam Quade, Richard Lloyd, and Trevor Volp.
Useful resources
The Beatsheet – northern region pest management newsletter
Best Bet IPM strategies and resources
References
Contact details
Dr Melina Miles
Queensland Department of Agriculture and Fisheries. 203 Tor St, Toowoomba.
0407113306
melina.miles@daf.qld.gov.au
@beatsheetblog
GRDC Project Code: DAQ00196,
Was this page helpful?
YOUR FEEDBACK