Glyphosate resistant annual ryegrass update - 2020 season
Author: Peter Boutsalis (University of Adelaide and Plant Science Consulting, Sam Kleeman (Plant Science Consulting) and Christopher Preston (University of Adelaide) | Date: 09 Feb 2021
Take home messages
- Glyphosate resistance in annual ryegrass continues to increase.
- There are ways to optimise glyphosate efficacy.
- Partner glyphosate with other herbicides to improve weed control.
Incidence of glyphosate resistance
The GRDC continues to support random weed surveys in cropping regions to monitor for changes in resistance levels in key weed species. The methodology involves collecting weed seeds from paddocks chosen randomly at pre-determined distances. Plants are tested in outdoor pot trials during the growing season. Resistance is defined as a sample where ³20% plant survival was detected in a pot trial. The incidence of glyphosate resistance identified in paddocks in different cropping regions across South Australia (SA) and Victoria (Vic) from random weed surveys is presented in Figure 1.
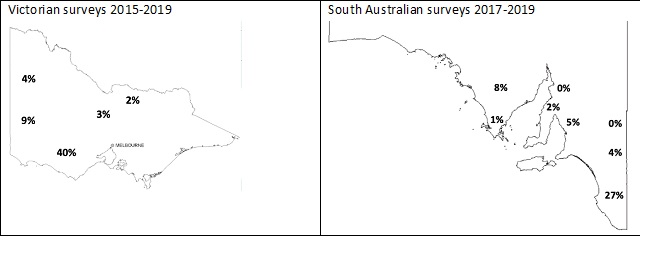
Figure 1. Incidence of paddocks containing glyphosate resistant ryegrass. Resistance is defined as a sample where ³20% plant survival was detected in a pot trial.
Additionally, Bayer CropScience Resistance Tracker provides access to a significant database, which contains data from commercial testing companies. This tool searches herbicide resistance for numerous weed species by postcode and year, with data collated over the past 15 to 20 years
2020 season
The early break in 2020 across most southern cropping regions resulted in an opportunity for knockdown weed control. Multiple applications of glyphosate and paraquat were possible targeting multiple flushes of weeds, in particular ryegrass from early autumn prior to sowing. Plants surviving following glyphosate application from Western Australia (WA), SA, Vic and New South Wales (NSW) were sent to Plant Science Consulting for testing using the Quick-Test method to verify whether herbicide resistance had contributed to survival in the field. The data presented in Figure 2 indicates that 43%, 70% and 79% of ryegrass samples sent from SA, Vic and NSW in 2020 respectively, were confirmed resistant to glyphosate. This highlights that in most cases, glyphosate resistance has contributed to reduced control in the paddock.
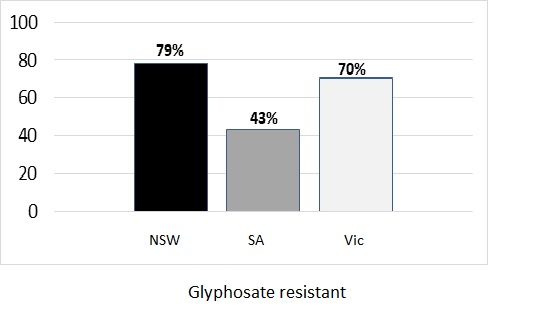
Figure 2. Percent (%) resistance to glyphosate confirmed in farmer ryegrass samples originating from 83 New South Wales, 37 South Australian and 74 Victorian cropping paddocks treated with glyphosate in autumn 2020. Testing conducted by Plant Science Consulting using the Quick-Test.
Discrepancy between resistance testing and paddock failures to glyphosate
In some cases, plants that have survived glyphosate application in the paddock are not resistant. Reasons for the discrepancy between paddock observations and a resistance test result can include poor application, application onto stressed plants, incorrect timing, sampling plants that were not exposed to glyphosate or a combination of the above.
Evolution of glyphosate resistance
Glyphosate was first registered in the 1970s and rapidly became the benchmark herbicide for non-selective weed control. Resistance was not detected until 1996 in annual ryegrass in an orchard in southern NSW (Powles et al. 1998). Only a few cases of resistance were detected in the following decade (refer to Bayer Resistance Tracker). The fact that it required decades of repeated use before resistance was confirmed indicated that the natural frequency of glyphosate resistance was initially very low. At the current time there are over a dozen species that have developed resistance to glyphosate in Australia, as shown in the CropLife herbicide resistant weed list. The most important species are ryegrass, sowthistle, barnyard grass and feathertop Rhodes grass. Ryegrass and sowthistle will be discussed further within this paper.
There are several contributing factors for the increasing glyphosate resistance in ryegrass with generally more than one factor responsible. Reducing rates can increase the development of resistance particularly in an obligate outcrossing species such as ryegrass resulting in the accumulation of weak resistance mechanisms to create individuals capable of surviving higher rates. This has been confirmed by Dr Chris Preston where ryegrass hybrids possessing multiple resistance mechanisms were generated by crossing parent plants with different resistance mechanisms.
Other factors that can select for glyphosate resistance by reducing efficacy include:
- Using low quality glyphosate products and surfactants.
- Mixing glyphosate with too many other active ingredients resulting in antagonism, particularly in low water volumes.
- Using low quality water, particularly hard water. Glyphosate is a weak acid, and therefore, binds to positive cations (e.g., magnesium, calcium and bicarbonate) that are in high concentration in hard water (i.e., >200 ppm),
- Applying glyphosate during periods of high temperature and low humidity, resulting in the rapid loss of glyphosate in solution from leaf surfaces, thereby reducing absorption.
- Applying glyphosate onto stressed plants can reduce translocation. Maximising glyphosate efficacy relies on translocation to the root and shoot tips. While this occurs readily in small seedlings, in larger plants, glyphosate is required to translocate further to the root and shoot tips to provide high levels of control.
- Shading effects that reduce leaf coverage resulting in sub-lethal effects.
- As glyphosate strongly binds to soil particles, application of glyphosate onto dust covered leaves can reduce efficacy.
- Application factors such as speed, nozzle selection and boom height can reduce the amount of glyphosate coverage.
- A combination of the above factors can reduce control, thereby increasing the selection for resistance.
Optimising glyphosate performance
The selection of glyphosate resistance can be reduced by considering the points mentioned previously. Additionally, there are a number of important pathways to follow to improve glyphosate performance including:
Avoid applying glyphosate under hot conditions. A trial spraying ryegrass during the end of a hot period and following a cool change was conducted in October 2019. Ryegrass growing in pots was sprayed at 8am, 1pm and 8pm with temperature and Delta T recorded prior to each application. Control of well hydrated plants ranged between 0% and 40% when glyphosate was applied during hot weather (30 to 32.5°C) and high Delta T (14 to 16.7) with the lowest control achieved when glyphosate was applied at midday (Figure 3). In contrast, glyphosate applied under cool conditions just after a hot spell resulted in significantly greater control (65%-80%), indicating that plants can rapidly recover from temperature stress provided moisture is not limiting, e.g., after rainfall.
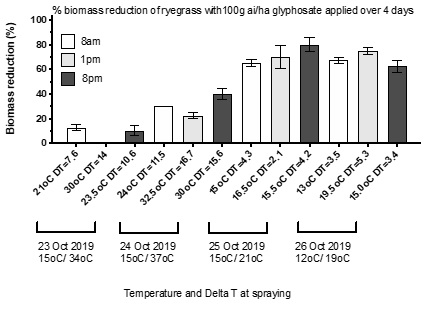
Figure 3. Effect of temperature and Delta T on glyphosate for ryegrass control.
Improving water quality and glyphosate activity by using ammonium sulfate (AMS). The addition of AMS has several functions. One is to soften water by combining to positively charged ions such as magnesium and calcium common in hard water. The negative charged sulphate ions combine with the positive cations preventing them from interacting with glyphosate and reducing its solubility and leaf penetration. Additionally, AMS has been shown to independently improve glyphosate performance, as the ammonium ions can work with glyphosate to increase leaf uptake. In a pot trial conducted with soft water, AMS was shown to significantly improve control of ryegrass with 222ml/ha (100g ai/ha) of glyphosate 450 (Figure 4). As a general rule, growers using rainwater (soft) should consider 1% AMS, if using hardwater (i.e., bore, dam water), 2% AMS is recommended. The addition of a wetter resulted in a further improvement in control.
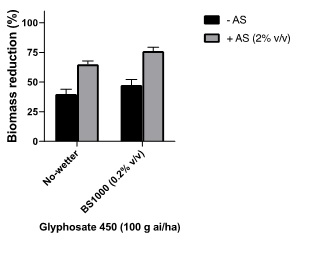
Figure 4. Effect of ammonium sulfate(AS) and wetter (BS1000) on glyphosate performance for ryegrass control.
Herbicide activity can vary at different growth stages. In a pot trial investigating the effect of glyphosate at four ryegrass growth stages (1-leaf to 4-tiller), good control was achieved at the three older growth stages but not on 1-leaf ryegrass (Figure 5). Most glyphosate labels do not recommend application of glyphosate on 1-leaf ryegrass seedlings because they are still relying on seed reserves for growth. Consequently, very little glyphosate moves towards the roots.
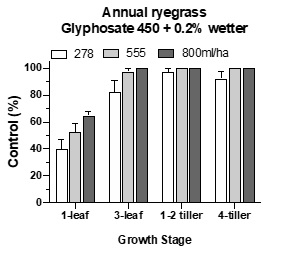
Figure 5. Effect of ryegrass growth stage on glyphosate activity.
A double knock strategy is defined as the sequential application of two weed control tactics to combat the same weed population. The most common double knock strategy is glyphosate followed by paraquat. It has been widely adopted to prevent or combat glyphosate resistance, particularly in ryegrass. The first ‘knock’ with glyphosate is aimed to control most of the population with the second ‘knock’ (paraquat) intended to kill any individuals that have survived glyphosate. In the presence of glyphosate resistance, paraquat applied one to five days following glyphosate was shown to provide optimum control in trial work conducted by Dr Christopher Preston (Figure 6). The timing depends on weed size and growing conditions, with three to five days required to maximise glyphosate activity. After a week (depending on environmental conditions) glyphosate resistant plants treated with glyphosate can stress, resulting in the absorption of less paraquat, reducing control with the second tactic. If growing conditions are poor or plants large, the stress imposed by glyphosate maybe further delayed.
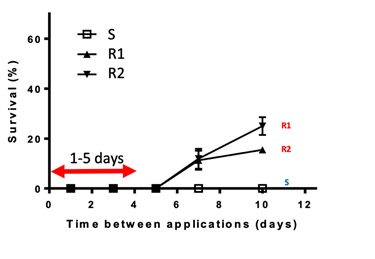
Figure 6. Double knock timing and its effect on ryegrass survival rate. Glyphosate applied onto a susceptible (S) and two glyphosate resistant ryegrass biotypes (R1 and R2) followed by paraquat 1, 3, 5, 7 and 10 days after application (DAA). (Source: Trial work conducted by Dr Christopher Preston (The University of Adelaide)).
Summary
In the southern cropping zone, glyphosate resistance in ryegrass continues to increase as indicated by random weed surveys across the region and the Bayer Resistance Tracker database. The early break in autumn 2020 resulted in the targeted testing of about 200 ryegrass populations prior to sowing with over half confirmed resistant to glyphosate. Although it took about 20 years after the registration of glyphosate for the first case of resistance to be confirmed, in the past 10 years there has been an exponential rise in the number of confirmed cases. Decades of strong selection pressure resulting from repeated use, coupled with application under suboptimum conditions has played a major role in the exponential rise. More efficient use of glyphosate combined with effective integrated weed management (IWM) strategies is required to reduce further increases in resistance.
Acknowledgements
The information for the random weed surveys was undertaken as part of GRDC project UCS00020, and therefore, is made possible by the significant contributions of growers through the support of the GRDC, the author would like to thank them for their continued support.
Contact details
Peter Boutsalis
Plant Science Consulting P/L
@PBoutsalis
University of Adelaide, Waite Campus, Glen Osmond SA 5064
peter.boutsalis@adelaide.edu.au
GRDC Project Code: UCS00020,
Was this page helpful?
YOUR FEEDBACK
