Canola disease update – what to look out for in 2021
Author: Kurt Lindbeck (NSW DPI, Wagga Wagga Agricultural Institute) and Steve Marcroft (Marcroft Grains Pathology P/L) | Date: 30 Jun 2021
Take home messages
- Diseases can be a significant constraint to canola production in Australia.
- In Australia, blackleg and sclerotinia stem rot are the most important and consistent diseases affecting canola production and have potential to cause significant yield loss.
- White leaf spot and powdery mildew are traditionally sporadic problems.
- Club root is not a widespread issue for canola producers in Australia, however the unique growing conditions and crop rotation in Tasmania could favour the disease.
- Where possible always follow integrated disease management (IDM) strategies to manage disease.
Background
Canola is an emerging crop in Tasmania. Attractive prices for product and fit within grain and graze farming systems is driving the interest in this crop within cropping rotations.
However, compared to mainland Australia there are differences in farming systems and rotations where canola is, and has potential to be grown in Tasmania. Some of these differences include sowing times (either as a winter sown or spring sown crop), rotation with other broadleaf crops (including other forage or culinary Brassica crops), variety choice and total area sown to the crop.
Diseases remain an important constraint to canola production. Experience from mainland Australia shows there are several important diseases affecting canola production that can cause significant yield loss and require an integrated approach to management. Key diseases that may affect canola production in Tasmania include:
- Blackleg – the most important and widespread disease of canola in Australia.
- Sclerotinia stem rot – an emerging production issue in medium to high rainfall zones.
- White leaf spot – a sporadic disease in southern production zones.
- Powdery mildew – important disease for northern NSW canola producers, sporadic in the south.
- Club root – detected very sporadically in NSW and Victoria, important disease in Canada.
Blackleg of canola
Causal Organism
Blackleg of canola is caused by the fungus, Leptosphaeria maculans, and is considered the most important disease of canola in Australia. The causal fungus is sexually reproducing, resulting in enormously diverse populations and therefore an ability to overcome resistance in Brassica napus (canola) cultivars. The fungal population has the potential to evolve very rapidly and responds quickly to selection pressures such as wide-scale sowing of canola cultivars with specific resistance genes.
Symptoms
Symptoms of blackleg first appear on cotyledons and the first true leaves. Lesions are round with a dark brown margin and grey centre. Pycnidia (small, pepper like spots) form within lesions. With rainfall events these secondary spores are splash dispersed within crops and further lesions develop on emerging leaves (secondary infection). The fungus then grows without symptoms through the vascular tissues to the crown where it causes a necrosis resulting in a dry rot (crown canker) at the base of the plant, often during or following stem elongation. Crown canker causes yield loss as it restricts water and nutrient uptake by the plant and will result in plants lodging prematurely and plant death.
In recent years changes in cropping systems have resulted in the appearance of upper canopy infection (UCI) symptoms in southern Australia. These appear as branch lesions, pod lesions, flower infections and raceme infections later in the season. Symptoms of UCI can also result in significant yield loss.
Disease Cycle
The blackleg pathogen survives and reproduces on the previous season’s canola stubble, producing airborne spores that infect newly emerging crops the following autumn/winter. This results in a very strong relationship between the intensity of canola production within a region and the level of blackleg development within commercial crops. Most ascospores are deposited within 500 - 600m of an old canola stubble. In Australia most ascospores are released from 12-month-old stubble from May to September.
Two-year-old stubble can be a significant source of inoculum, especially in tight canola rotations. Canola crops are most susceptible to seedling infection up to the 6-leaf stage. Infections formed after the 6-leaf stage have a significantly reduced chance of forming a stem canker. During the growing season in southern Australia, crops are subjected to ascospore showers from nearby stubble and spread of pycnidia within crops. Following harvest, the fungus colonises old stubble residue, forming pseudothecia that release ascospores the following season.
In Tasmania, consideration should also be given to other Brassica crops that are grown in rotation or in close proximity to canola, as these crops can also host the blackleg fungus.
Management
Blackleg can be minimised using a number of tools. Spores of the blackleg fungus are released from the previous year’s canola stubble, so an increased area of canola results in increased disease pressure. The most effective blackleg management tool is to keep a 500 m distance from the current season’s crop and last year’s canola stubble. Practices that accelerate the decomposition of canola stubble can also assist in reducing the level of spore release.
Sowing of cultivars with high blackleg resistance is also very important, however be aware that sowing the same cultivar for three or more years will result in the erosion of resistance with time, as the pathogen population adapts to overcome the resistance. All canola cultivars are now classified into different resistance groups, enabling the rotation of canola cultivars with different resistance genes. A range of fungicides are on the market that can be applied to seed and foliage. It is strongly recommended to rotate actives where possible to avoid the development of resistance in the pathogen population. Croplife Australia has on-line resources available for rotating fungicides in canola available here.
Another useful resource is the BlacklegCM app. Prior to sowing use the BlacklegCM decision support tool to identify high risk paddocks and explore management strategies to reduce yield loss due to disease.
Sclerotinia stem rot
Causal Organism
The fungus that causes Sclerotinia stem rot is called Sclerotinia sclerotiorum. This fungus can infect over 300 plant species, mostly broadleaf plants, including many crop, pasture and weed species. This includes plants like canola, other Brassica crops, pulses, sunflower, lucerne, cape weed, and shepherds’ purse. The main feature of the sclerotinia stem rot fungus is the production of hard, black, survival bodies on infected plant tissue called sclerotia which enable the fungus to survive for up to 8 years in the field.
Symptoms
The most striking symptom of Sclerotinia stem rot is the formation of stem lesions on plants during flowering. Stem lesions start as discrete infections initiated by a lodged petal against the stem or branch junction. Lesions continue to develop under favourable conditions as elongated, bleached infections, sometimes with darker rings, showing the stepwise development of the fungus. As conditions dry out, lesions become brittle and result in stem breakage. Within the pith of the stem small, black survival structures (sclerotia) form. Under wet conditions fluffy, white mycelium and sclerotia can form on the outside of stem lesions. Infected plants will appear dead within crops. These can be entire plants or branches that shatter prematurely, causing yield loss.
Disease Cycle
Sclerotia residing in soil soften and form an apothecia (a small, golf tee shaped structures, 5 – 10 mm in diameter) after a period of prolonged soil wetness (in southern Australia this often occurs around the end of June). Only sclerotia in the top few centimetres of the soil will germinate and produce apothecia. Sclerotia that are buried deeper will remain dormant for extended periods and may germinate if moved closer to the soil surface. Most sclerotia will remain viable for up to 3 – 4 years then survival slowly declines.
Spores of the Sclerotinia pathogen cannot infect canola leaves and stems directly. They require petals as a food source for spores to germinate, grow and colonise the petal. When the petal eventually drops, it may become lodged in a leaf axil or at branch junctions along the stem. If conditions are moist the fungus grows out of the petal and invades healthy plant stem tissue which will result in a stem lesion and production of further sclerotia within the stem which will be returned to the soil after harvest. Sclerotia also have the ability to germinate in the soil, produce mycelium and directly infect canola plants in close proximity, causing a basal infection. These plants are identified in the field by having infection at the stem base and often infection extending into the upper taproot. In the past this type of infection was generally rare.
Weather conditions during flowering play a major role in determining the development of the disease. The presence of moisture during flowering and petal fall will determine if Sclerotinia stem rot develops. Dry conditions during this time can quickly prevent development of the disease, hence even if flower petals are infected, dry conditions during petal fall will prevent stem infection development
Management
Currently, there are no commercial canola cultivars available on the Australian market with resistance to Sclerotinia stem rot. Management of the disease relies on the use of cultural and chemical methods of control. Crops that flower early in the season, in late winter and early spring are more prone to developing stem rot, as high moisture favours disease development. Where possible canola crops should flower in spring when conditions are warmer and drier. Foliar fungicides should be considered in those districts which are at a high risk of disease development (eg. districts where the disease frequently occurs, long flowering period and reliable spring rainfall).
Here are several foliar fungicides currently registered for use in Australia to manage Sclerotinia stem rot including Aviator Xpro® (bixafen 75 g/L + prothioconazole 150 g/L), Prosaro® (prothioconazole 210 g/L + tebuconazole 210 g/L), Veritas® (tebuconazole 200 g/L + azoxystrobin 120 g/L) and products containing procymidone or iprodione. To maximise the economic benefits from foliar fungicides consider the factors that lead to disease development. All three factors (host, pathogen and environment) need to coincide for the disease to develop. The diagram below shows those factors that lead to Sclerotinia stem rot development.
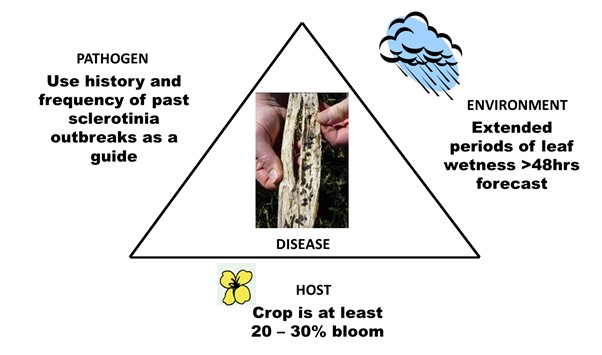
Figure 1. Factors that lead to stem rot development.
Points to consider when using a foliar fungicide to manage Sclerotinia stem rot
- The most yield loss from Sclerotinia occurs from early infection events. Early infection is likely to result in premature ripening of plants that produce little or no yield.
- Plants become highly susceptible to infection once flowering commences. Research in Australia and Canada has shown that an application of foliar fungicide around the 20% - 30% bloom stage (20% bloom is 14 – 16 flowers on the main stem, 30% bloom is approx. 20 flowers on the main stem) can be effective in significantly reducing the level of Sclerotinia stem infection.
- The objective of the fungicide application is to prevent early infection of petals while ensuring that fungicide also penetrates into the lower crop canopy to protect potential infection sites (such as lower leaves, leaf axils and stems). Timing of fungicide application is critical.
- A foliar fungicide application is most effective when applied before an infection event (eg. before a rain event during flowering). These fungicides are best applied as protectants and have no curative activity.
- In general, foliar fungicides offer a period of protection of up to 3 weeks. After this time the protectant activity of the fungicide is compromised. In some crops, development of lateral branch infections later in the season is not uncommon if conditions favourable for the disease continue. The greatest yield loss occurs when the main stem becomes infected, especially early. Lateral branch infection does cause yield loss, but at a much reduced level.
- Use high water rates and fine droplet sizes for good canopy penetration and coverage.
- Fungicide choice is often secondary to timing of application.
White leaf spot
Causal Organism
White leaf spot is caused by the fungus Mycosphaerella capsellae, which can affect a wide range of Brassica crops. The disease has a history of being sporadic in Australia, with disease severity varying significantly between seasons.
Symptoms
The most common symptom of white leaf spot are leaf lesions. These start as small, necrotic, grey-brown spots (1-2mm diameter) on the tips and edges of leaves. These spots develop into larger round, tan coloured lesions (5-10mm diameter). The initial dark spot will often remain in the centre of the lesion and can be a diagnostic feature. As lesions mature, they become lighter in colour from pale grey to white, with a dark tan-brown margin. Under favourable conditions lesions can coalesce on leaves, spreading to most of the leaf.
Disease Cycle
The fungus survives between seasons on infected canola (or Brassica crop) stubble. When conditions become favourable, airborne spores are released from previous years’ stubble and land on new season crops, causing leaf infections. Once established within the crop, conidia are splash dispersed and cause infections on surrounding leaves and plants. Later in the season, stems and pods can become infected.
Management
Currently management options for white leaf spot are limited. Miravis® (pydiflumetofen) is currently registered for use on canola to manage the disease at the vegetative stage. There are currently no canola cultivars known to have resistance. Similar to recommendations for blackleg management, avoid sowing this season’s crop near old canola or Brassica crop stubble which could harbour the fungus.
Powdery mildew
Causal Organism
The fungus that causes powdery mildew is Erysiphe cruciferarum, requiring a living host in order to grow and reproduce. It grows on the surface of plants, penetrating epidermal cells of the host to obtain nutrients. In some regions of Australia powdery mildew is a sporadic disease or develops late in the season and has no impact on production, while the disease can hinder crop production and harvesting operations in regions like northern NSW.
Symptoms
The initial symptoms of powdery mildew appear as whitish patches on the upper and lower surfaces of leaves and stems. These white patches (mycelium) expand quickly under favourable conditions and form a dense, white layer that resembles talcum powder. The layer can be readily rubbed off with a finger. Often purple blotching my form as a result of infection.
Disease Cycle
Most powdery mildew outbreaks occur in Australia in spring (early or late – depending on location). Moderate daily temperatures, with low relative humidity in combination with cool night temperatures that promote dew formation, favour disease development. Cool temperatures and wet weather do not favour the disease. The fungus overwinters on living hosts or as cleistothecia on stubble. Often a ‘green bridge’, such as infected crop or weed host will provide a mode of survival between seasons in Australia.
Management
There are no known canola cultivars resistant to powdery mildew. There are currently no fungicides registered in Australia to manage powdery mildew of canola.
Club root
Causal Organism
Clubroot is a highly sporadic disease of canola in Australia and is caused by the fungus Plasmodiophora brassicae. The pathogen can attack the roots of a wide range of Brassica species, affecting yield and quality. The pathogen is very long lived, surviving for up to 20 years within infested soils. This disease is currently a major production issue for canola producers in Canada.
Symptoms
The most common symptom of clubroot is swelling of roots and hypocotyl. Canola roots can form a mass of galls when infected. The extent of galling depends in plant age, root morphology, and period of infection. From above infected plants appear to be stunted, with fewer and smaller leaves. Foliage may be blue-green and wilted, even when soil water is available, due to water and nutrient uptake being impeded by gall formation. Premature death of plants can occur. Early in the season, galls are firm and white inside, later turning brown and rotting.
Disease Cycle
Resting spores of the fungus remain viable in the soil for years. In the presence of suitable host plants, the resting spores germinate, form spores that swim and attach to root hairs. The spores continue to invade root cells and multiply to produce further spores that either penetrate deeper into the host root or are released back into surrounding soil to cause other infections. As the infection develops, long lived resting spores are formed within infected root tissue, which are released passively back into the soil as infected root tissue dies and decays.
Management
In Canada there are currently several management options available to producers. These include growing a canola cultivar resistant to the disease, following a strict crop rotation and allowing a significant break between crop hosts, minimising activities where soil may be transferred on machinery into new area and controlling weed hosts.
Conclusion
The area sown to canola in Tasmania has the potential to increase due to the popularity of the crop within farming systems. Experience from south-eastern Australia has shown there to be several important diseases that affect canola production namely, blackleg and Sclerotinia. Both diseases have increased in significance due to increased frequency of canola in rotations. The farming system in Tasmania is unique where canola may be grown in rotation with other Brassica crops, which have the potential to harbour canola pathogens.
Crop rotation with non-host crops, avoiding sowing canola adjacent to last season’s canola or Brassica crop stubble, variety selection/ rotation, and strategic use of fungicides are all effective tools that can be used to minimise the impact of disease. The decision to apply fungicides is not always clear cut and should be based on the disease risk profile of the crop and scouting for disease symptoms during the growing season.
Where possible an integrated approach should be used to manage diseases that utilises non-chemical and chemical approaches.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support.
Useful resources
NSW Department of Primary Industries – Winter Crop Variety Sowing Guide 2021
Blackleg Management Guide 2020
Contact details
Kurt Lindbeck
NSW Department of Primary Industries, Wagga Wagga Agricultural Institute
02 69 381 608
kurt.lindbeck@dpi.nsw.gov.au
Steve Marcroft
Marcroft Grains Pathology
Grains Innovation Park, Horsham
03 53 812 294
steve@grainspathology.com.au
GRDC Project Code: UOM1904-004RTX, UM00051, CSP00187, MGP1905-001SAX, BLG206,
Was this page helpful?
YOUR FEEDBACK
