Cereal disease update 2022: Septoria of wheat, net blotch of barley and fungicide resistance
Author: Mark Mclean, Hari Dadu & Grant Hollaway (Agriculture Victoria, Horsham) Fran Lopez Ruiz (Centre for Crop and Disease Management, Curtin University) | Date: 14 Jul 2022
Take home messages
- Septoria tritici blotch caused yield loss in highly susceptible varieties in the MRZ (Wimmera) but not the LRZ (Mallee). During 2021, fungicides did not provide an economic increase in grain yield in either environment.
- Net form of net blotch is common and can cause substantial grain yield and quality loss in susceptible varieties, where yield potential exceeds 4t/ha.
- Decision support apps are available to assist with fungicide application decisions for stripe rust and yellow leaf spot in wheat during the growing season.
- Development of fungicide resistance is increasing in cereal pathogens but can be slowed through the adoption of integrated control strategies and prudent use of fungicides.
Septoria in wheat
Septoria tritici blotch (STB) (Zymoseptoria tritici) is a damaging disease of wheat with large losses known to occur in susceptible cultivars in high rainfall cropping zones (HRZ) of Victoria and South Australia. However, the impact of STB on yield in the medium (MRZ) and low rainfall cropping zones (LRZ) in Victoria and South Australia is less understood, even though this disease has become common in these regions. The increase in STB prevalence is associated with increased use of cultivars susceptible to STB and stubble retention practices. There is much uncertainty about the impact and control of this disease in medium and low rainfall zones.
To determine yield loss of STB in Victoria and South Australia’s MRZ and LRZ, GRDC is supporting a new investment in research led by Agriculture Victoria, in partnership with the South Australia Research and Development Institute (SARDI). This investment will investigate the epidemiological conditions required and the impacts of STB in these regions to inform disease management decisions.
In Victoria, seven field experiments were conducted during 2021: three each in the MRZ (Longerenong) and LRZ (Watchupga, with Birchip Cropping Group) and one experiment in the HRZ (Hamilton) of Victoria to understand the conditions that suit the disease progression, study the impact of STB on wheat with different resistance ratings and identify optimal timing for fungicide application during seasons at risk. Similar experiments occurred in South Australia.
Conditions critical for STB development and spread
At three locations in Victoria (LRZ, MRZ and HRZ), susceptible wheat inoculated with infected stubble were grown. The plots were monitored for disease development and a Pessl weather station (courtesy of ADAMA) collected climatic data that influences disease progress including temperature, relative humidity, precipitation, and leaf wetness.
STB severity was different in each of the three locations. As expected, disease development was greatest in the HRZ and least in the LRZ (Figure 1). The three locations showed different weather conditions (Table 1). Low maximum temperatures combined with high growing season rainfall distributed evenly across the season and larger periods of leaf wetness at Hamilton provided ideal conditions for STB progress and the potential for maximum impact (Table 1). Conditions at Longerenong and Watchupga were only partially or not conducive respectively for STB development and wheat varieties with moderate resistance grown in these conditions are likely to escape yield losses.
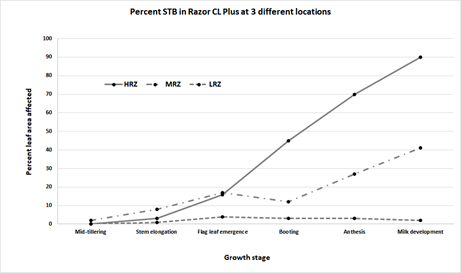
Figure 1. STB severity (% leaf area affected) across time in wheat (cv. Razor CL Plus, susceptible to STB) at three different locations in Victoria during 2021.
Table 1: Summary of weather at three locations in Victoria during the 2021 growing season.
Location | Growing season (April to October) | |||
|---|---|---|---|---|
Total rainfall (mm) | Mean leaf wetness (hrs/day) | Mean maximum temperature (°C) | Mean number of rain days/month# | |
Watchupga (LRZ) | 172 | 10 | 19 | 11 |
Longerenong (MRZ) | 262 | 7 | 17 | 14 |
Hamilton (HRZ) | 419 | 20 | 15 | 18 |
#A rainy day is defined as a day with a rainfall of at least 0.1mm or more.
Yield loss due to STB
Two yield loss experiments, one each at Longerenong (MRZ) and Watchupga (LRZ), were conducted during 2021. Each experiment had six commercial wheat varieties with different resistance/susceptibility to STB, and six replications of plus and minus disease treatments.
Good levels of STB developed at the Longerenong site (MRZ) in the susceptible varieties Scepter (S) and LRPB Impala (SVS) which caused 8% and 7% of yield loss, respectively (Table 2). This was due to suitable conditions during the season for the disease to progress, with above average rainfall during the spring that supported infection of the top three leaves during grain fill, but also shows that partial resistance can provide adequate disease suppression in this environment.
Table 2: Septoria tritici blotch severity (% leaf area affected) and grain yield of six wheat varieties at Longerenong, Victoria with high (Max) and low (Min) disease, during 2021.
Variety | Rating | Disease severity (% leaf area affected) | Grain yield (t/ha) | Yield loss (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
29-Jul, Z25-31 | 31-Aug, Z37 | 25-Oct, Z69-75 | ||||||||
Max.c | Min. | Max. | Min. | Max. | Min. | Max. | Min. | |||
Sunlamb | MR | 0 | 0 | 5 | 2** | 0 | 0 | 5.2 | 5.1 | - |
LRPB Orion | MRMS | 1 | 0 | 1 | 1 | 8 | 0** | 5.2 | 5.3 | - |
LRPB Lancer | MS | 1 | 1 | 7 | 4** | 6 | 0** | 5.6 | 5.8 | - |
Hammer CL Plus | MSS | 2 | 1* | 12 | 7** | 11 | 0** | 5.3 | 5.3 | - |
Scepter | S | 2 | 1** | 24 | 12** | 49 | 1** | 6.2 | 6.8** | 8 |
LRPB Impala | SVS | 2 | 1** | 27 | 14** | 56 | 5** | 5.3 | 5.8** | 7 |
**=statistically significant at 1% Lsd; *=statistically significant at 5% Lsd.
a Max. = Maximum disease; Min. = Minimum disease.
In contrast, conditions were not conducive for STB at Watchupga (LRZ) where only low levels of STB developed and yield loss was not measured, even in the susceptible varieties Scepter and LRPB Impala (Table 3).
Table 3: Septoria tritici blotch severity (% leaf area affected) and grain yield of six wheat varieties at Watchupga, Victoria with high (Max) and low (Min) disease, during 2021.
Variety | Rating | Disease severity (% leaf area affected) | Grain yield (t/ha) | ||||||
|---|---|---|---|---|---|---|---|---|---|
28-July, Z25-31 | 2-Sep, Z39-49 | 06-Oct, Z61-71 | |||||||
Max.a | Min. | Max. | Min. | Max. | Min. | Max. | Min. | ||
Sunlamb | MR | 0 | 0ns | 1 | 0ns | 0 | 0ns | 3.0 | 3.2ns |
LRPB Orion | MRMS | 0 | 0ns | 0 | 0ns | 1 | 1ns | 2.7 | 3.0ns |
LRPB Lancer | MS | 0 | 0ns | 2 | 1** | 1 | 0** | 2.7 | 2.5ns |
Hammer CL Plus | MSS | 0 | 0ns | 4 | 2** | 2 | 1** | 2.8 | 3.0ns |
Scepter | S | 1 | 0ns | 9 | 3** | 4 | 1** | 3.0 | 3.2ns |
LRPB Impala | SVS | 1 | 0** | 10 | 4** | 4 | 2** | 2.9 | 2.8ns |
**=statistically significant at 1% Lsd; *=statistically significant at 5% Lsd, ns=not significant at 5%.
a Max. disease = Maximum disease; Min. disease = Minimum disease.
Relative STB severity in each variety corresponded with resistance status at both locations. The suppression in STB levels in the partially resistant varieties demonstrates that avoiding susceptible varieties in these regions should be adequate to manage STB.
Fungicide timing for STB management
Two experiments, one each at Longerenong (MRZ) and Watchupga (LRZ), were conducted to determine the optimal fungicide timing for STB control. Six replications of six fungicide treatments consisting of either single or combinations of seed and/or foliar applied fungicide and an untreated control (UTC) were applied to a susceptible variety, Scepter (S). As limited disease developed at the low rainfall site, only the results from Longerenong will be discussed here.
At Longerenong, all fungicide treatments reduced STB severity compared to the UTC, but the early applications of seed only or a single spray at Z31 were not as effective as any of the three treatments that included a fungicide application at Z39 (Table 4).
Table 4: Septoria tritici blotch severity (% leaf area affected) and grain yield of wheat (cv. Scepter (S)) in response to different fungicide treatments in the Victorian medium rainfall zone (MRZ), Victoria during 2021.
Treatment | Active ingredient | Disease severity (% leaf area affected) | Grain yield (t/ha) | ||
|---|---|---|---|---|---|
29-July, Z31* | 31-Aug, Z37* | 25-Oct, Z73# | |||
Seed | Fluquinconazole | 1a | 13a | 34c | 6.0 |
Foliar at Z31 | Benzovindiflupyr + Propiconazole | 2b | 13a | 17b | 6.1 |
Foliar at Z31 and Z39 | Benzovindiflupyr + Propiconazole at Z31 and Epoxiconazole at Z39 | 2b | 13a | 1a | 6.2 |
Seed and Foliar at Z39 | Fluquinconazole as seed + Epoxiconazole at Z39 | 1a | 11a | 3a | 6.2 |
Foliar at Z39 | Epoxiconazole | 2b | 23b | 3a | 6.1 |
Untreated control | - | 2b | 23b | 52d | 6.1 |
P | 0.00 | <0.001 | <0.001 | 0.47 | |
Lsd (0.01) | 1.14 | 2.72 | 4.65 | ns | |
Means with one letter in common are not significant *Average of single plot assessments; #Average of the top three leaves of ten tillers per plot. | |||||
Although fungicide application reduced STB severity significantly, no significant yield benefit was observed with any of the fungicide treatments (Table 4).
These trials showed that fungicide applications may not be economical in either the LRZ or MRZ during years with below average to average rainfall conditions. Economical returns may be possible, particularly in the MRZ should above average rainfall conditions occur, and the fungicide strategy will need to reflect seasonal conditions.
Net form of net blotch of barley
Net form of net blotch (NFNB) is a common foliar disease of barley in Victoria due to the adoption of susceptible varieties such as RGT Planet and Spartacus CL. During 2022, the risk of loss due to NFNB will be greatest where susceptible varieties are sown into barley stubble from either of the last two years. NFNB is seed and wind-borne, and therefore can establish in crops where there is no recent paddock history of barley. As a result, any susceptible crop should be monitored if seasonal conditions are favourable. A NFNB strain virulent on Spartacus CL is now well established in Victoria. The Centre for Crop and Disease Management (CCDM) researchers have identified that this strain is also generally resistant to fluxapyroxad (a member of the Succinate Dehydrogenase Inhibitors (SDHI) group of fungicides), the active ingredient in the seed treatment Systiva®. Reduced sensitivity toward triazoles such as tebuconazole and propiconazole have also been found to be increasing in frequency. Subsequently these fungicides may no longer be effective for NFNB control (see section on fungicide resistance below for more details). It also means that Systiva resistant NFNB can be found in other varieties such as Compass, Commodus and Maximus. Note that these varieties have effective host-plant resistance to NFNB and are unlikely to have losses from NFNB infection.
It is also important to monitor for infections from other diseases, such as the spot form of net blotch (SFNB), and scald in varieties such as RGT and Spartacus CL, as these can also be damaging if not controlled in favourable seasons.
Fungicides will be an important part of NFNB control in susceptible varieties. Previous research has shown that two fungicide applications can be effective for NFNB management. Dual foliar fungicide application strategies have been most effective with the first application at Z25-31, and the second at Z39-55. Earlier applications tend to provide the majority of suppression in shorter season environments and later applications in longer, high rainfall environments. It is important to rotate fungicides with different modes of action to ensure effective suppression and to minimise the chance of further resistance developing. This strategy will also be very effective for other common barley diseases such as SFNB, scald and leaf rust.
In general, varieties susceptible to NFNB with a yield potential of 5t/ha or more are at risk of economic losses, especially during seasons with a wet spring. Experiments conducted in the Victorian Mallee and Wimmera during 2019 demonstrated that when NFNB was severe, with up to 70% of leaf area affected at grain fill (Figure 2), there was up to 18% grain yield loss and reductions to grain plumpness (Tables 5, 6 and 7) in the very susceptible (VS) breeding line VB9613. The partially resistant varieties had less infection and grain yield loss than VB9613. RGT Planet, rated susceptible to very-susceptible (SVS), had up to 11% infection and 5% grain yield loss, while all other varieties had less than 5% leaf area infected at the end of the season. This illustrated that varieties rated as moderately susceptible (MS) or better, had little chance of loss in the medium to low rainfall environment.
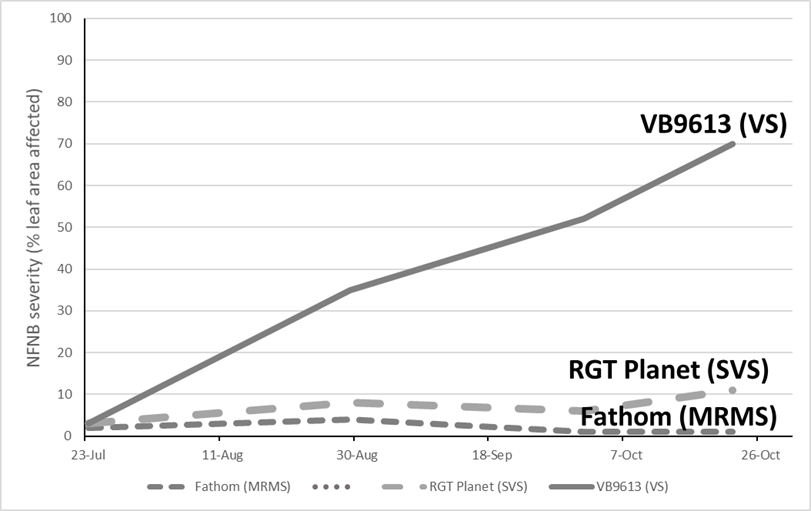
Figure 2. Severe net form of net blotch infection developed in breeding line VB9613, rated susceptible to very-susceptible (SVS), compared to moderate and low infection in RGT Planet (SVS) and Fathom moderately resistant to moderately susceptible (MRMS) at Birchip during 2019.
Table 5: Net form of net blotch severity (% leaf area affected) and grain yield of eight barley varieties grown at Birchip during 2019.
Variety | Rating# | Disease severity (%) 22 October 2019 (Z85) | Grain Yield (t/ha) | |||||
|---|---|---|---|---|---|---|---|---|
NFNB | SFNB | Dis.A | Fung. | % Loss | ||||
Banks | MR | 1 | 5 | 5.6 | 5.5ns | 0 | ||
Fathom | MRMS | 1 | 0 | 5.3 | 5.4ns | 2 | ||
Commander | MS | 1 | 5 | 4.9 | 5 ns | 2 | ||
SakuraStar | MS | 1 | 0 | 5.4 | 5.5ns | 2 | ||
Spartacus CL | MSS | 1 | 10 | 6.2 | 6.4ns | 3 | ||
LG Alestar | S | 3 | 0 | 5.3 | 5.6* | 5 | ||
RGT Planet | SVS | 11 | 10 | 6.5 | 6.7 ns | 3 | ||
VB9613 | VS | 70 | 0 | 4.2 | 5.1* | 18 | ||
P= | <0.001 | - | - | - | - | |||
Lsd (0.05) = | 2.9 | - | - | - | - | |||
ADis. = Disease - 1kg infected stubble, no fungicides; Fung. = no stubble, Systiva + Prosaro® at Z31 and Z39.
* = Significant at 5%; ns = not statistically significant when the fungicide and disease treatments are compared.
# rating = moderately resistant (MR), moderately susceptible (MS), moderately resistant – moderately susceptible (MRMS), moderately susceptible – susceptible (MSS), susceptible (S), susceptible – very susceptible (SVS), very susceptible (VS).
Table 6: Effect of net form of net blotch on grain quality for eight barley varieties grown at Birchip during 2019.
Variety | Rating# | Screenings | Retention | ||||||
|---|---|---|---|---|---|---|---|---|---|
Dis.A | Fung.B | % Increase | Dis. A | Fung.B | % Loss | ||||
Banks | MR | 3 | 5ns | 0 | 76 | 77ns | 1 | ||
Fathom | MRMS | 2 | 2ns | 0 | 91 | 90ns | 0 | ||
Commander | MS | 3 | 4ns | 1 | 84 | 83ns | 0 | ||
SakuraStar | MS | 5 | 4ns | 1 | 76 | 79ns | 3 | ||
Spartacus CL | MSS | 3 | 2ns | 1 | 76 | 79ns | 3 | ||
LG Alestar | S | 4 | 3ns | 1 | 76 | 78ns | 2 | ||
RGT Planet | SVS | 3 | 4ns | 0 | 75 | 73ns | 0 | ||
VB9613 | VS | 10 | 5* | 5 | 28 | 45* | 17 | ||
ADis. = Disease - 1kg infected stubble, no fungicides; BFung. = no stubble, Systiva + Prosaro at Z31 and Z39.
* = Significant at 5%; ns = not statistically significant when the fungicide and disease treatments are compared.
# Rating = moderately resistant (MR), moderately susceptible (MS), moderately resistant – moderately susceptible (MRMS), moderately susceptible – susceptible (MSS), susceptible (S), susceptible – very susceptible (SVS), very susceptible (VS).
Table 7: Net form of net blotch severity, frost damage and grain yield of eight barley varieties grown at Horsham during 2019.
Variety | Rating# | NFNB severity (%LAA) | Frost damage | Grain yield (t/ha) | Loss (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
22 October 2019 Z85 | (%) | Dis.A | Fung.B | ||||||
Banks | MR | 1 | 5 | 5.5 | 5.5 | 0 | |||
Fathom | MRMS | 1 | 5 | 6.3 | 6.5 | 3 | |||
Commander | MS | 3 | 30 | 5.7 | 5.5 | 0 | |||
SakuraStar | MS | 1 | 5 | 5.9 | 5.9 | 0 | |||
Spartacus CL | MSS | 1 | 2 | 6.3 | 6.4 | 2 | |||
LG Alestar | S | 2 | 8 | 5.2 | 5.0 | 0 | |||
RGT Planet | SVS | 9 | 2 | 5.7 | 6.0* | 5 | |||
VB9613 | VS | 47 | 2 | 4.8 | 5.2** | 8 | |||
P= | <0.001 | - | - | - | |||||
Lsd (0.05) = | 3.2 | - | - | - | |||||
ADis. = Disease - 1kg infected stubble, no fungicides; BFung. = no stubble, Systiva + Prosaro at Z31 and Z39.
** = statistically significant at 5%, * = Significant at 5%; ns = not statistically significant when the fungicide and disease treatments are compared.
# Rating = moderately resistant (MR), moderately susceptible (MS), moderately resistant – moderately susceptible (MRMS), moderately susceptible – susceptible (MSS), susceptible (S), susceptible – very susceptible (SVS), very susceptible (VS).
Fungicide resistance in Victoria
Resistance to fungicides is becoming an increasing threat to cereal crops across Australia. The status of resistance to fungicides in important cereal diseases is summarised in Table 8 and is based on work by the Fungicide Resistance Group (FRG) at the Centre for Crop and Disease Management (CCDM at Curtin University).
Table 8: Fungicide resistance and reduced sensitivity cases identified in Victorian and South Australian wheat and barley crops.
Disease | Pathogen | Fungicide Group | StatusA | Industry implications |
|---|---|---|---|---|
Barley powdery mildew | Blumeria graminis f.sp. hordei | 3 (DMI) | Lab detection | May see some reduced efficacy in field. Field resistance detected in WA |
Wheat powdery mildew | Blumeria graminis f.sp. tritici | 3 (DMI) | Field Resistance | Some Group 3 DMIs will be ineffective in field |
11 (QoI) | Field Resistance | Group 11 fungicides ineffective | ||
Barley net-form of net blotch | Pyrenophora teres f.sp. teres | 3 (DMI) | Reduced sensitivity | Expect to see reduced field performance with time |
7 (SDHI) | Field resistance (SA) Lab detection (Vic) | Increasing occurrences of field failure expected | ||
Barley spot-form of net blotch | Pyrenophora teres f.sp. maculata | 3 (DMI) | Lab detection | Products still effective but will decline as resistance develops |
7 (SDHI) | Not detected | Field resistance detected in Western Australia but not eastern Australia | ||
Wheat septoria tritici blotch leaf blotch | Zymoseptoria tritici | 3 (DMI) | Reduced sensitivity | May see some reduced efficacy in field |
11 (QoI) | Field resistance (SA) | Two detections in SA during 2020, but not detected in 23 locations across Victoria and South Australia in 2021 |
ALab detection - Measurable differences in sensitivity of the pathogen to the fungicide when tested in the laboratory. Detection of resistance in the lab can often be made before the fungicide’s performance is impacted in the field; Reduced sensitivity – Some reduction in fungicide performance which may not be noticed in the field. Serves as a warning that resistance is developing in the pathogen. Increased fungicide rates as per registered labels may be necessary. Field Resistance - Fungicide fails to provide an acceptable level of control of the target pathogen at full label rates.
Following are the latest findings on fungicide resistance in Victoria.
Barley net blotches
On the South Australian Yorke Peninsula during 2019, a mutation in the net form of net blotch (NFNB) pathogen was identified that conferred field resistance to SDHI (Group 7) fungicides which includes key actives such as fluxapyroxad and bixafen. Limited testing during 2021 detected this mutation in six barley samples from Victoria suggesting that this resistance may be widespread across the southern region (Table 6). Mutations in NFNB that confer reduced sensitivity (partial resistance) to SDHI fungicides were also common in the Victorian samples.
Table 9: Mutations associated with resistance and reduced sensitivity to demethylase inhibitor (DMI, Group 3) and succinate dehydrogenase inhibitor (SDHI, Group 7) fungicides detected in net blotch samples from Victoria and South Australia in 2021.
Location | State | Disease present | DMI Reduced Sensitivity | SDHI Resistance | SDHI Reduced Sensitivity | ||||
|---|---|---|---|---|---|---|---|---|---|
Promoter lindel | F489L | PtSdhC-H134R | PtSdhD-D145G | PtmSdhC-N75S | |||||
SFNB | NFNB | NFNB | NFNB | NFNB | |||||
n/a | Vic | NFNB+SFNB | - | + | + | - | - | ||
Banyena | Vic | NFNB | - | + | + | - | + | ||
Banyena | Vic | NFNB | - | + | + | - | + | ||
Minyip | Vic | NFNB+SFNB | - | + | + | + | - | ||
Minyip | Vic | NFNB+SFNB | - | + | + | - | - | ||
Horsham | Vic | NFNB+SFNB | - | + | - | - | - | ||
Horsham | Vic | NFNB | - | + | + | + | - | ||
Minlaton | SA | NFNB+SFNB | - | + | - | - | - | ||
Minlaton | SA | NFNB+SFNB | - | - | - | - | - | ||
Minlaton | SA | NFNB+SFNB | + | - | - | - | - | ||
Minlaton | SA | NFNB+SFNB | - | - | + | - | + | ||
Minlaton | SA | SFNB | - | - | - | - | - | ||
A mutation that confers reduced sensitivity to the DMI (Group 3) fungicides was also common in the limited testing conducted on Victorian NFNB samples during 2021. Previously, this mutation was observed in NFNB from the Yorke Peninsula of SA and is associated with reduced sensitivity to DMI compounds. These types of mutations provide an early warning that the pathogen is accruing mutations that will eventually enable the pathogen to be resistant to the fungicide.
Within the spot form of net blotch testing, a mutation that confers partial resistance to DMI (Group 3) fungicides was identified in South Australia but not Victoria. This mutation has been detected in Western Australia since 2016 and is associated with reduced sensitivity to some DMI compounds. This detection in eastern Australia provides an early warning that practices are required to slow the development and spread of fungicide resistance.
Septoria tritici blotch
Based on studies conducted several years ago by NSW DPI, it is known that reduced sensitivity (partial resistance) to the triazole (DMI, Group 3) fungicides is well established within the Victorian STB pathogen population.
The finding of resistance mutations to strobilurin fungicides (Qol, Group 11) in two STB pathogen samples collected in South Australia during 2020 was very concerning for growers in the southern region. In vitro testing of these isolates showed a 200-fold increase in resistance to azoxystrobin compared with the susceptible strain. Fortunately, testing of 32 samples collected from Victoria, Southern Australia and New South Wales during 2021 did not detect this mutation suggesting that this mutation is still at a low prevalence. However, this re-enforces the need to adopt strategies to protect the limited range of fungicides that we have available (see below).
Wheat powdery mildew
Resistance to both DMI (Group 3) and Qol (Group 11) fungicides has been detected in Victoria. Analysis of samples from seven paddocks in north-east Victoria were all positive for the mutation associated with resistance to Qol fungicides. This means there will be increasing occurrences of field failure in areas prone to powdery mildew and where susceptible varieties are grown.
Fungicide resistance management
There are five strategies that growers can adopt to slow the development of resistance in pathogen populations and therefore extend the longevity of the limited range of fungicides available.
- Avoid susceptible crop varieties. Where possible, select the most resistant crops suitable and/or avoid putting susceptible crops in high-risk paddocks.
- Rotate crops. Avoid planting crops back into their own stubble or adjacent to their own stubble.
- Use non-chemical control methods to reduce disease pressure. Delaying sowing, early grazing are examples of strategies that can reduce disease pressure.
- Spray only if necessary and apply strategically. Avoid prophylactic spraying and spray before disease gets out of control.
- Rotate and mix fungicides/modes of action. Use fungicide mixtures formulated with more than one mode of action, do not use the same active ingredient more than once within a season and always adhere to label recommendations.
For more information on the management of fungicide resistance consult the “Fungicide Resistance Management Guide” available from AFREN.
Apps to support in-crop disease management decisions
Apps are available to assist in making decisions around disease management in crop. The apps use models that produce predictions based on information on variety resistance rating, plant growth stage, yield potential and the presence of disease inoculum. The model predictions are compared and validated with field trial data to ensure accuracy and reliability. The models were developed by DPIRD and GRDC with input from AgVic. The apps are available free from the Apple App Store and Google Play.
StripeRustWM App
The StripeRustWM App is available to download on tablets. The app can support decision making around fungicide use for stripe rust management during the season. The app uses information that is specific to a local area or paddock to improve accuracy. Comparisons to field trials have demonstrated a high level of accuracy and reliability.
YellowSpotWM App
The YellowSpotWM App was released during 2021 and is available to download on tablets and smartphones. Its predictions are being developed in line with data from the 2021 season, ready for the 2022 growing season. This app will support in-crop fungicide decisions.
NetBlotchBM App
The NetblotchBM tool will be tested during 2022 and the app will be released during 2023 to help support in-crop fungicide management decisions for the spot and net forms of net blotch in barley. If you would like to help with testing of NetBlotchBM, please contact Anna Hepworth (anna.hepworth@dpird.wa.gov.au), or any of the lead authors of this paper.
Conclusion
In the absence of proactive disease control, yield losses due to diseases can be greater than 20%. The risk from Septoria and net blotches is likely to be greater with a wet season (La Niña). It is, therefore, important that strategies are proactive to effectively manage cereal diseases this season. Disease management plans should consider paddock history, variety, yield potential and appropriate use of fungicides.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support.
Funding for this work was provided by the Victorian Government (Agriculture Victoria) and the GRDC through the GRDC projects: DJPR2104-004RTX, DJP2103-005RTX, DJP2003-011RTX, DAW1810-007RTX, CUR1905-001SAX, CUR00023.
Thanks to Agriculture Victoria’s Cereal Pathology Team: Jordan McDonald, Glenn Sluggett, Joshua Fanning, Melissa Cook, Luise Fanning, Bhanu Kalia and Chloe Findley. Thanks also to the Birchip Cropping Group for field trials within the Victorian Mallee and to our research collaborators Andrew Milgate (NSW DPI), Julian Taylor (University of Adelaide) and Tara Garrard (SARDI).
Useful resources
Agriculture Victoria cereal disease guide
Field Crop Diseases Victoria 577545
Contact details
Grant Hollaway
Agriculture Victoria
Private Bag 260, Horsham VIC 3401
03 4344 3111
grant.hollaway@agriculture.vic.gov.au
@Grant_Hollaway
Mark McLean
Agriculture Victoria
Private Bag 260, Horsham VIC 3401
03 4344 3111
mark.s.mclean@agriculture.vic.gov.au
@msmclean777
Hari Dadu
Agriculture Victoria
Private Bag 260, Horsham VIC 3401
03 4344 3111
Hari.Dadu@agriculture.vic.gov.au
@Imharidadu
GRDC Project Code: DJP2104-004RTX, DJP2103-005RTX, DJP2003-011RTX, DAW1810-007RTX, CUR1905-001SAX, CUR1403-002BLX,
Was this page helpful?
YOUR FEEDBACK
