Rust in 2023 and beyond – pathotypes and varieties and strategies for durable deployment of new genes for resistance
Take home messages
- Stripe rust in particular is likely to be important again in 2023; monitor for the presence of the green bridge, and if present, make sure it is destroyed at least 4 weeks before crops are sown, either by heavy grazing or herbicides
- The structure of stripe rust populations in eastern Australia has become more complex in recent years. This has changed the stripe rust response of many varieties of common wheat, durum wheat and triticale, stressing the need for close monitoring of varieties rated S or above and being prepared to apply fungicides if needed.
- Five incursions of stripe rust have been documented since it was first detected in Australia in 1979 (Ding et al. 2021). Three originated from Europe (1979, 2017 and 2018) and one North America (2002). Each has cost the industry hundreds of millions of dollars; for example, it was estimated that between $40-$90 million was spent on fungicides annually in 2003, 2004 and 2005 following the second 2002 incursion (Wellings, 2007). The critical importance of thoroughly laundering clothing and personal effects after interstate or overseas travel cannot be overstated.
- Insensitivity to DMI fungicides has been detected experimentally in the leaf rust pathogens of barley (nationally) and wheat (eastern Australia). Please monitor barley and wheat crops that have been sprayed for leaf rust and notify us of the success or otherwise of the treatment.
- The variability of rusts and their rapid spread across the Australian continent reinforces the importance of regular and nationally coordinated monitoring of these pathogens. All stakeholders are encouraged to monitor crops, barley grass and wild oat for rust throughout 2023, and to forward freshly collected samples in paper only to the Australian Cereal Rust Survey, at University of Sydney, Australian Rust Survey, Reply Paid 88076, Narellan NSW 2567.
Wheat stripe rust pathotype update
Cereal rust pathotypes (aka races, strains) are isolates of rust that differ in ability to overcome the resistance genes in cereal varieties. They are identified by using a field-collected sample of rust to infect a set of cereal varieties (‘differentials’), each carrying a known resistance gene, and determining which resistance genes are overcome and which are not. This process takes about 3 weeks. Given favourable conditions for rust development, the pathotype/s present is a major determinant of how varieties perform and whether or not yield loss will occur.
Knowing what pathotypes are present, their distribution and impact on cultivars is the foundation of all rust control. This information is used to:
- monitor the effectiveness of resistance genes in cereal varieties
- interpret and determine varietal rust response
- provide new or relevant rust pathotypes for breeding and research
- understand how new pathotypes develop
- understand pathogenic and genetic variability, and the evolutionary potential of rust pathogen populations.
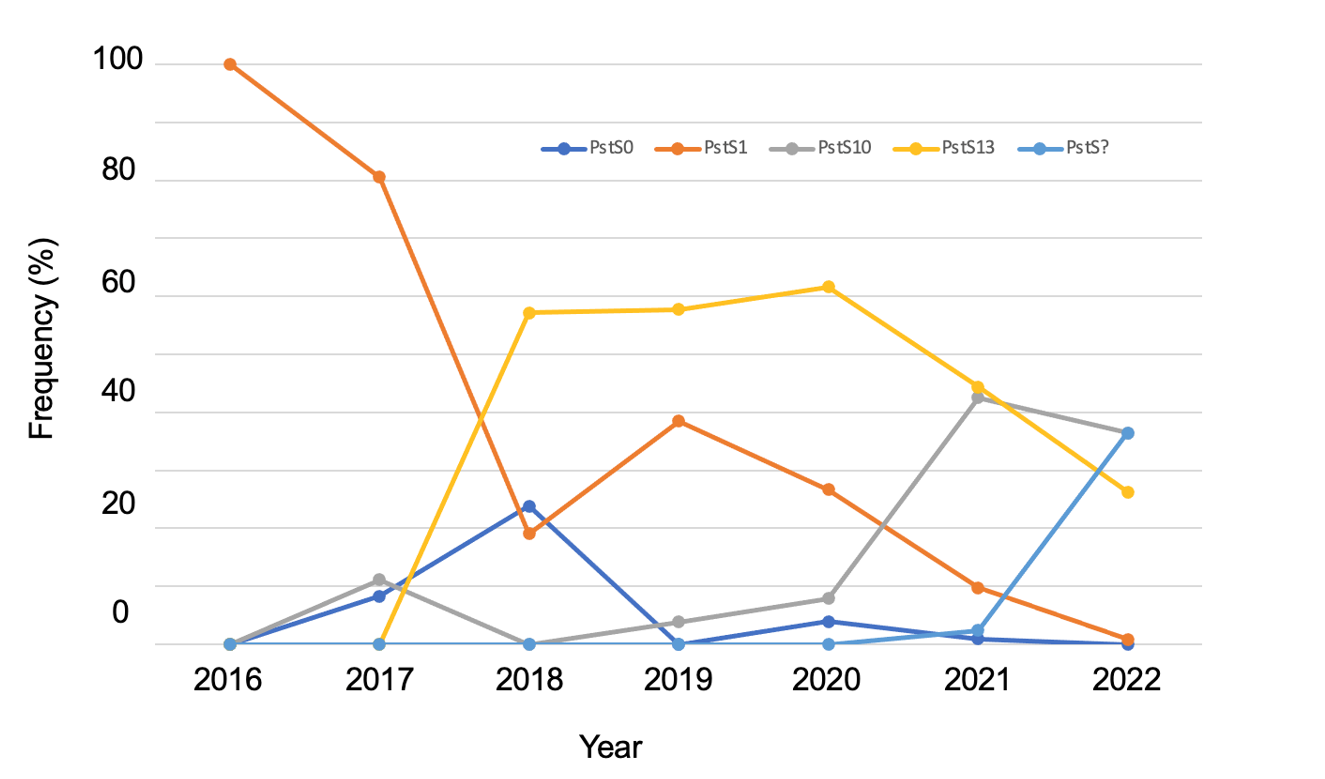
Epidemics of wheat stripe rust in eastern Australia in 2020, 2021 and 2022 were caused almost entirely by two pathotypes that found their way into Australia, from probably Europe/South America, in 2017 and 2018. These two pathotypes belong to two genetic groups, defined by internationally accepted Multi Locus Genotypes (‘MLGs’) based on DNA fingerprinting markers: PstS10 (pathotype 239 E237 A- 17+ 33+; ‘239’; 2017); PstS13 (pathotype 198 E16 A+ J+ T+ 17+; ‘198’; 2018). In 2022, these two pathotypes, along with a third pathotype of unknown MLG (pathotype 238 E191 A+ 17+ 33+; ‘238’) that was first detected in 2021, were responsible for the extensive and damaging stripe rust epidemic experienced.
Figure 1 depicts the relative frequencies of all wheat stripe rust pathotypes detected annually since 2016, including the two previously detected MLG pathotype groups PstS0 (first detected in 1979, originating from Europe) and PstS1 (first detected in 2002, originating from North America; aka the ‘WA’ pathotype group). Of note in 2022 was the rapid increase in frequency of pathotype ‘238’ (PstS?) after its initial detection in 2021, and reductions in the frequencies of pathotypes belonging to the other four MLGs. Our greenhouse tests are yet to detect any virulence advantage of pathotype 238 over the other groups, meaning that its increase in frequency in 2022 is likely due to increased ‘aggressiveness’ – for example, faster growing, producing more spores.
Figure 1. Frequency (%) of four internationally accepted DNA fingerprint MLG groups (PstS0, PstS1, PstS10, PstS13) of wheat stripe rust pathotypes, and a fifth as yet undefined group (PstS?) in eastern Australia, 2016 through 2022.
The expression of adult plant resistance (APR)
Seasonal conditions not only affect the stripe rust pathogen, they also affect crop development and expression of resistance genes in different wheat varieties. Most varieties rely on adult plant resistance (APR) genes for protection from stripe rust, which as the name implies, become active as the plant ages. Consequently, all varieties, unless rated resistant (R), are susceptible as seedlings and move towards increasing resistance as they develop and APR genes become active.
Much remains to be known about the expression of APR. The growth stage at which APR becomes active differs between wheat varieties and relates to their resistance rating. An MR variety would generally have APR active by GS 30–GS 32 (early stem elongation), MR-MS by GS 37–GS 39 (flag leaf emergence), MS by GS 49–GS 60 (awn peep-start of flowering) and MSS by GS 61–GS 75 (flowering to mid-milk). Varieties rated S or worse have relatively weak levels of resistance that are generally of limited value in disease management. Note that a variety can have a higher or lower resistance rating to individual pathotypes of the pathogen, depending on its resistance genes and the corresponding virulence of different stripe rust pathotypes.
Mild temperatures during 2021 and 2022 that extended well into spring slowed crop development, which consequently delayed the expression of APR genes whilst also favouring multiple cycles of stripe rust infections. This extended the time between growth stages and affected management strategies, which in more susceptible varieties is based around early protection with fungicides until APR within a variety is reliably expressed.
Higher levels of nitrogen nutrition can also delay crop maturity and expression of APR genes within varieties whilst also being more conducive to stripe rust infection (thicker canopy and leaf nitrate food source for pathogen). Differences in nitrogen nutrition can relate to rotation history (pulse vs cereal/canola in previous season) and rate and timing of fertiliser application (pre-sowing, at sowing or in-crop). However, under higher levels of N nutrition, the resistance level of a variety only ever drops by one category; it does not for instance make a MR/MS variety an S. Under high levels of N nutrition, growers need to manage a variety as one category lower in resistance (that is, manage a MR/MS as an MS).
Fungicide insensitivity/resistance in rust
The use of fungicides in Australian broadacre farming since the early 1980s has resulted in the emergence of fungal pathogen isolates with insensitivity to them, especially DMI fungicides. This has been well documented in, for example, septoria tritici blotch, wheat powdery mildew, barley powdery mildew, and net form of net blotch, and in blackleg in canola.
Cases of fungicide insensitivity in rust pathogens are fortunately much less common. Apart from reports from Brazil of a decline in the field performance of DMIs against the Asian soybean rust pathogen, few if any agronomically important cases of fungicide insensitivity in a rust pathogen are known.
We tested more than 800 rust isolates of wheat (stem rust, leaf rust, stripe rust), barley (leaf rust) and oat (crown rust, stem rust) for sensitivity to the DMI fungicide tebuconazole under controlled conditions. Importantly, these tests revealed insensitivity in isolates of the leaf rust pathogens of barley (Puccinia hordei) and wheat (Puccinia triticina) collected in 2021 to not only tebuconazole, but also epiconazole, prothioconazole, propiconazole and triadimenol. While tebuconazole is not registered for the control of leaf rust in barley, it is registered for scald and mildew control in barley (maximum rate 290 mL/ha) and for rust diseases in wheat and oat (maximum rate 290 mL/ha).
More extensive testing using standard historical isolates of both rust pathogens from our rust collection revealed that in P. hordei, insensitivity occurs in a clonal lineage of pathotypes that trace back to an exotic incursion into WA that was first detected in 2001. All isolates within this lineage that we tested, including the original 2001 isolate, were insensitive to tebuconazole at rates of more than six times the maximum rate of 290mL/ha recommended for rust control in wheat and oat. Insensitive isolates are common in all Australian barley growing regions.
Within the wheat leaf rust pathogen P. triticina, insensitivity to the four DMI fungicides was identified in a single pathotype, namely 93-3,4,7,10,12 +Lr37, which could grow and sporulate on leaves treated with rates of tebuconazole up to 25 times the recommended high field application of 290 mL/ha. This pathotype was first detected in southern NSW in October 2020 and is considered to be of exotic origin. It was isolated again in 2021 and 2022, and although it increased in frequency and has spread to Victoria and Queensland, it remains at low levels in the overall P. triticina population.
Our work appears to be the first documented case of insensitivity to a fungicide in a cereal attacking rust pathogen. Further in-field testing of these findings needs to be undertaken and at this stage there have been no reports of in-field failures of fungicides associated with cereal rust insensitivity. However, it reminds us of the remarkable abilities of these pathogens to change and adapt to circumvent the strategies used to control them, be they genetic resistance or agrochemicals.
Broader threats posed by cereal rust pathogens
Ongoing frequent changes in cereal rust pathogens, well documented by our rust surveillance over the past 10 years, have presented new challenges to resistance breeding and in crop rust control. They include:
- loss of important resistance genes in wheat, barley, oat and triticale, due to local mutations (for example, Rph3 and Rph7 in barley, Yr27 in wheat, Pc91 in oat)
- more frequent east-to-west spread of new rust pathotypes within Australia, resulting in new virulences in the west that have rendered varieties susceptible (for example, Lr13, Lr27+31)
- introductions of exotic wheat leaf rust pathotypes in 2014 (from North America) and 2020 (source currently unknown)
- introductions of two exotic wheat stripe rust pathotypes in 2017 (Europe) and 2018 (Europe or South America)
- local emergence of two genetically divergent stripe rust isolates in 2021, one that infects wheat and one with increased virulence on barley
- emergence and spread of fungicide insensitivity in the leaf rust pathogens of barley (national) and wheat (eastern Australia).
These new rusts have reduced profitability for growers of wheat (bread and durum), barley, oat and triticale. The loss of genetic resistance has also impacted breeding programs, slowing genetic gain with an anticipated knock-on effect to grower profitability in the years ahead. Combined, they highlight the need for ongoing RD&E to ensure effective and timely industry-wide rust protection.
Strategies for durable deployment of new genes for resistance
The term durable resistance is sometimes mistakenly equated with enduring rust control in agriculture. Clearly, growing only varieties that carry high levels of durable resistance at a large scale would be expected to provide enduring rust control across agro-ecological zones, continents and possibly beyond. However, it is important to appreciate that resistance that has proven durable may not remain effective forever, stressing the importance of genetic diversity in the resistances deployed.
The durability of resistance genes when deployed over large areas is complex, being determined not just by the ability of the pathogen to acquire matching virulence, but also other traits in the pathogen and host that can impact on overall disease epidemiology. For example, on the pathogen side, our long-term surveys of pathogenicity of cereal rust pathogens in Australia have provided many examples where certain pathogen genotypes seem to have greater fitness, which is independent of virulence for resistance genes (such as the recent example of wheat stripe rust pathotype ‘238’). On the host side, a change to growing early maturing wheat varieties developed by William Farrer in Australia had a huge impact in reducing losses to stem rust through ‘disease escape’. Both of these factors can influence the overall size of the pathogen population, and in so doing, affect the timing of epidemic onset, disease pressure on varieties carrying incomplete levels of resistance, and how frequently virulent mutant pathotypes emerge.
In view of this complexity, diversity of genetic resistance must be seen as a key ingredient in large scale enduring control of plant diseases. It has been argued that even where specific or major resistance genes are used, diversity in the resistance genes deployed insures against lack of durability and hence reduces genetic vulnerability. Above all, responsible use of resistance genes, which relies on knowing what resistance genes are present in varieties and breeding populations, and monitoring pathogen populations with respect to deployed resistances, are crucial in ensuring that the genetic bases of resistances are not narrowed.
Conclusion
The confirmation of two further incursions of the wheat stripe rust pathogen brings to four the number documented since this disease was first detected in Australia in 1979. The evidence available implicates Europe as the source of three of these incursions (1979, 2017 and 2018) and North America as the source of the other one (2002). Along with the two exotic incursions of the wheat leaf rust pathogen detected in 2014 and 2020, this continues the trend that has emerged from our long-term pathogenicity surveys of cereal rusts of an increasing frequency of exotic incursions with time, presumably associated with increased international movement of people and inadvertent transport of rust spores on contaminated clothing. Exotic wheat rust incursions have cost the industry hundreds of millions of dollars. The importance of thoroughly laundering clothing and personal effects after interstate or overseas travel cannot be emphasised enough.
Stripe rust was very common and damaging in wheat crops in eastern Australia during the 2022 season, and there were many situations in which fungicides were used to control the disease. This was in part due to the occurrence of pathotype 198 E16 A+ J+ T+ 17+. The amount of stripe rust that developed was, however, nowhere near that caused by this pathotype in Argentina in 2016/17 and 2017/18. The much lower impact of pathotype 198 in Australia compared to its impact in Argentina and Europe is a clear endorsement of the value of genetic resistance in controlling rust diseases in cereals, and of the efforts of all stakeholders in using genetics as the foundation of rust control here in Australia.
The latest responses of Australian wheat and triticale cultivars to the pathotypes reported here, based on detailed greenhouse and field testing, are provided in our Cereal Rust Report (Volume 19 Issue 1,released August 2022), which can be downloaded from our website.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. The national rust pathotype surveillance program, conducted by staff at the University of Sydney, involves active participation by many people including state-based regional cereal pathologists, scientists in universities and in the private sector, grain growers, and their critical contributions are gratefully acknowledged.
References
Ding Y, Cuddy WS, Wellings CR, Zhang P, Thach T, Hovmøller MS, Qutob D, Brar GS, Kutcher HR, Park RF (2021) Incursions of divergent genotypes, evolution of virulence and host jumps shape a continental clonal population of the stripe rust pathogen Puccinia striiformis. Molecular Ecology.
Wellings CR (2007) Puccinia striiformis f. sp. tritici in Australia: a review of the incursion, evolution and adaptation of stripe rust in the period 1979-2006. Australian Journal of Agricultural Research 58: 567-575.
Useful resources
McIntosh RA, Wellings CR, Park RF (1995). ‘Wheat rusts: an atlas of resistance genes.’ (CSIRO Publishing: Melbourne)
Contact details
Robert F. Park
The University of Sydney
Plant Breeding Institute
107 Cobbitty Road, Cobbitty NSW 2570
Ph: 02 9351 8806
Email: [email protected]
Date published
August 2023
GRDC Project Code: UOS2207-0002RTX,
Was this page helpful?
YOUR FEEDBACK