Maximising the fixed nitrogen benefits of pulses
Author: Ross Ballard, Elizabeth Farquharson (South Australian Research and Development Institute), Maarten Ryder, Judy Rathjen and Matthew Denton (University of Adelaide). | Date: 07 Feb 2017
Take home messages
- Field peas fixed on average 16.6kg N/t shoot dry matter (DM). There is room for improvement.
- There are differences in symbiotic performance of different pea varieties.
- New acid tolerant strains of rhizobia have performed well on beans.
- Mixing rhizobia with other agricultural products can result in nodulation failures.
Background
The 600,000ha of pulse crops grown in the GRDC Southern Region contribute approx. 70,000t of fixed nitrogen (N) into the farming system, worth approx. $50 million per year. In this region, field peas, faba beans and lentils make up almost 80% of the pulse crop area. A feature common to these three pulses (and also vetch) is that they are nodulated by the same species of rhizobia and so to some extent face the same symbiotic challenges, notwithstanding their obvious differences in dry matter (DM) production and N demand.
Where these pulses have been successfully grown on neutral or alkaline soils, the likelihood of inoculation response is low, so efforts to improve N2-fixation are being directed at understanding the contribution of host genotypes and how paddock conditions limit nodulation and N2-fixation.
The other scenario is where the legume host has not previously been grown or where soil conditions are not conducive to long term persistence of their rhizobia. Here, efforts to improve N-fixation are being directed at the development of improved inoculants (for beans on very acid soils) and assessing the compatibility of rhizobia with agricultural chemicals and fertilisers, so that nodulation failures are avoided.
This paper provides a summary of the symbiotic performance of field peas in contemporary farming systems and the potential for symbiotic improvement. The performance of both commercial and new bean and pea rhizobia on very acidic soils is examined and examples provided of the potential pitfalls of mixing rhizobia with other agricultural products.
Methods
Twenty-five field pea trials were sown across South Australia (SA) and Victoria (Vic) from 2011 to 2016 to assess the performance of the pea symbiosis and identify opportunities for improvement. Variation in N-fixation between pea varieties, response to inoculation and the impact of agronomic practices including ‘starter’ N were examined. Five bean trials are also reported that specifically examine the performance of rhizobia strains selected for improved acidity tolerance.
Nodulation (approximately eight weeks after sowing), shoot DM production at mid pod fill, nitrogen (N) fixation (15N natural abundance method), grain yield and %N in the grain were determined. The number and N-fixation capacity of the rhizobia present in the soil at the sites was determined using greenhouse bio-assays. All references to soil pH are based on its measurement using CaCl2.
Laboratory work examined the compatibility of rhizobia used in commercial inoculants with various pesticides, fertilisers, trace elements and organic amendments. In the example provided, survival of rhizobia when mixed with a commercial zinc sulphate (ZnSO4) solution was tested in flasks in laboratory conditions, to mimic field tank mixing. The scaled down test measured rhizobial survival in the 6 hour period after mixing by dilution plating of the samples and counting the number of rhizobial colonies that grew.
Results and discussion
What is the potential for symbiotic improvement?
Substantial variation occurred in the symbiotic performance of field peas across 23 field sites. Whilst the upper boundary for fixed N was approx. 22kg/t shoot DM, which is consistent with the levels reported by Unkovich et al. (2010), many observations fell well below this level, indicating scope for improvement. On average, 16.6kg N/t shoot DM was fixed with the majority of the data sitting between 22kg and 10kg fixed N/t shoot DM. Almost a third of the values were less than 15kg fixed N/t shoot DM, including some observations below the 10kg boundary line. The values do not include N contained in the root components.
Figure 1. Relationship between total shoot DM production (measured at mid pod fill) and the amount of N fixed in shoots (kg/ha) by field peas at 23 of the field sites. Lines indicate kg of fixed N/t shoot DM.
Factors contributing to this variation are examined and opportunities for improvement through legume selection, inoculation and the management of soil conditions are discussed.
What can be done to improve fixed N?
Legume selection
The data in Figure 1 reinforce the importance of legume DM production to the potential amount of N that is fixed. Choosing the legume with the best growth potential for the paddock and optimising conditions for its growth should therefore take priority to achieve the highest N-fixation.
For field peas, differences in symbiotic performance between varieties (Table 1) provide an opportunity to increase the amount of fixed N added to the farming system. As previously indicated, because DM production sets the upper boundary for fixed N, conventional forms of varieties (for example, Coogee, Percy and Parafield) that produce the most shoot DM, generally fix more total N and leave more N behind in the stubble because of their lower harvest index. Across the range of pea lines examined, N fixed/t shoot DM varied by 5kg (14 to 19), total N fixed/ha by 26kg (79 to 105) and fixed N remaining in the stubble by 52kg (27 to 79). PBA Hayman was excellent where seasonal and disease conditions suited its growth, however its performance was not consistent.
The differences in symbiotic performance were not solely due to DM production, with differences in % Ndfa also measured (Table 1).
Table 1. Measures of fixed N in different field pea varieties and breeding lines (means of seven trials).
Variety | Form | Fixed N | Ndfa | Fixed N Shoots and Pods | Fixed N Stubble | Compatibility with soil rhizobia (% of inoculant strain) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
(kg/t shoot DM) | (%) | (kg/ha) | (kg/ha) | |||||||
Mean | Best Site | Mean | Best Site | Mean | Best Site | Mean | Best Site | |||
PBA Coogee | C-T | 19 | 24 | 75 | 91 | 99 | 168 | 62 | 116 | 45 |
PBA Percy | C-T | 19 | 30 | 73 | 91 | 102 | 178 | 62 | 180 | 51 |
Parafield | C-T | 18 | 23 | 76 | 91 | 105 | 154 | 58 | 102 | 72 |
Morgan | SL-T | 17 | 21 | 71 | 91 | 98 | 136 | 53 | 72 | 66 |
OZP1208 | SL-MT | 17 | 22 | 61 | 82 | 93 | 155 | 43 | 91 | 76 |
Kaspa | SL-MT | 17 | 23 | 73 | 92 | 99 | 166 | 45 | 101 | 63 |
PBA Hayman | C-T | 17 | 23 | 72 | 97 | 102 | 176 | 79 | 128 | 59 |
PBA Pearl | SL-MT | 16 | 21 | 70 | 89 | 98 | 144 | 40 | 81 | 54 |
Wharton | SL-MT | 16 | 24 | 56 | 74 | 85 | 144 | 44 | 77 | 58 |
PBA Gunyah | SL-MT | 16 | 21 | 75 | 95 | 95 | 157 | 34 | 80 | 53 |
PBA Oura | SL-MT | 16 | 22 | 65 | 84 | 88 | 145 | 37 | 88 | 66 |
PBA Twilight | SL-MT | 15 | 24 | 67 | 87 | 88 | 174 | 35 | 97 | 62 |
OZP1101 | SL-T | 15 | 21 | 69 | 86 | 93 | 149 | 27 | 67 | 65 |
OZP1104 | SL-MT | 15 | 21 | 70 | 88 | 90 | 168 | 39 | 92 | 62 |
OZP0903 | SL-MT | 14 | 20 | 60 | 86 | 79 | 123 | 31 | 65 | 77 |
LSD (P<0.05) | 2 | 3 | 4 | 11 | 8 | 20 | 9 | 17 | 15 | |
*C=conventional leaf type, SL=semi-leafless, T= tall growth form, MT=medium-tall growth form. | ||||||||||
Greenhouse tests have shown that the average N-fixation capacity of naturalised soil rhizobia is 62% when compared to the commercial inoculant strains and that there is also variation in this value among pea varieties. Interestingly, Coogee was found to form the least effective association (45%) with the rhizobia in 10 soils, even though it has been one of the best and most consistent performers in the field. This contradiction might be explained by the high number of nodules formed by this variety at some sites (up to 119 nodules per plant), indicating the plant is able to compensate to some extent in the field.
Differences in the N-fixation potential of the pea genotypes will be incorporated into an on-line tool that will provide growers with estimates of N produced and left behind by the different varieties.
Strategic use of inoculants and the development of better strains of rhizobia
Inoculation provides one of the most cost effective ways to improve legume performance where the legume (or another from the same inoculation group) has not previously been grown and/or where conditions are detrimental to the survival of rhizobia in the soil.
Chickpeas and lupins are typically responsive to inoculation in the GRDC Southern Region because they have been less widely grown and their rhizobial requirement is more specific than for some other legume species. The first time they are grown, nodulation can often be improved by increasing the rate of inoculation.
Peas, beans, lentils and vetch are nodulated by the same species of rhizobia and have been widely grown, so many soils support adequate populations of rhizobia for these legumes. Nonetheless, inoculation of these legumes is still necessary where they are grown on acid soils for the first time. Even if they have been previously grown, there is still a moderate chance of inoculation response on acidic soils (<pH 6.0) because the rhizobia from previous crops may not persist at a high enough number for optimal nodulation.
The general relationship between soil pH and the number of pea rhizobia in soil shown in Figure 2 illustrates that rhizobial number declines below pH 6.0 to approx. 100 rhizobia/g soil, where there is a moderate to high chance of response to inoculation.
Figure 2. Relationship between soil pH (0-10cm, 0.01 M CaCl2) and the most probable number of pea nodulating rhizobia persisting in the soil. Modified from Drew et al. (2012).
Most of the observations below 10kg fixed N/shoot DM, previously described in Figure 1, were measured at Riverton in SA, the site with lowest pH (4.7).
Work to select strains of bean and pea rhizobia with improved acid tolerance is well progressed. New strains, including some isolates from the Riverton site, were initially screened for their ability to form nodules in very low pH (4.2) hydroponic solutions. The best strain produced 20-fold more nodules than the commercial rhizobia. Field evaluation commenced in 2015 (beans on Kangaroo Island) and following encouraging results (Table 2) was continued in 2016. In the initial small plot trial, two new strains of rhizobia were significantly better at nodulating broad beans than the current commercial strain — nodulation ratings were higher and more uniform. In addition, shoot N and fixed N were almost doubled (Table 2). In another grower run trial (replicated four times), SRDI954 again produced more nodules than WSM1455, increased grain yields by 8% and the amount of N fixed by more than 40kg/ha.
Table 2. Effect of inoculation treatment on the nodulation and N2-fixation of Kareema broad beans in acidic soil (pH 4.9) on Kangaroo Island, SA in 2015.
Treatment | Nodulation score* | Total shoot N (kg/ha) | Fixed shoot N (kg/ha) |
|---|---|---|---|
SRDI954 | 3.28 a | 117 | 95 |
SRDI970 (syn. Vetch W181) | 3.17 ab | 125 | 111 |
Granular Tag Team | 2.72 abc | 93 | 71 |
Group F rhizobia (WSM1455) | 1.46 cde | 61 | 52 |
Not inoculated | 1.22 de | 79 | 59 |
Grower bean crop surrounding trial | 1.96 (n/a) | ||
* Nodulation scores: 0 to 5, based on Corbin et al. (1977). Nodulation scores followed by the same letter are not significantly different at P<0.05. | |||
The new rhizobial strains were tested at three other locations and also on peas in 2016 in fully replicated field trials (Table 3). The sites were below pH 5.0 and responsive to inoculation, due to the absence of naturalised rhizobia. New strains, including SRDI954, improved the nodulation of beans, but not field peas. Strain SRDI970 was the top ranked strain for grain yields at both Kangaroo Island and Wanilla, with Ballyrogan still to be harvested. Persistence of the rhizobia in the soils over summer will be determined and is expected to provide further discrimination among the strains.
Table 3. Nodule weight (mg DM) and grain yield (t/ha) of Samira faba beans and Kaspa field peas at acidic field sites sown in 2016.
Kangaroo Is., SA Bean pH 4.8 | Ballyrogan, Vic Bean pH 4.9 | Wanilla, SA Pea pH 4.6 | ||||
|---|---|---|---|---|---|---|
Nodule weight mg DM | Grain yield t/ha | Nodule weight mg DM | Grain yield t/ha | Nodule weight mg DM | Grain yield t/ha | |
No rhizobia | 143 | 2.8 | 12 | pending | 103 | 1.9 |
Commercial strain | 594 | 4.1 | 312 | pending | 360 | 2.3 |
SRDI954 | 879 | 4.2 | 426 | pending | 262 | 2.4 |
SRDI969 | 745 | 4.7 | 425 | pending | 260 | 2.3 |
SRDI970 | 744 | 4.8 | 408 | pending | 252 | 2.5 |
LSD (0.05) | 214 | 0.9 | 156 | - | 89 | 0.2 |
A strain with improved acid tolerance for beans remains our primary focus, since faba and broad beans are increasingly being grown on very acid soils in the high rainfall areas in the GRDC Southern Region. The new rhizobial strains have so far performed well with beans on soils below pH 5.0 and at the very least are worthy of further evaluation. A separate cohort of strains with improved acid tolerance for peas has been selected and is being evaluated by colleagues from WA (Ron Yates, Department of Agriculture and Food WA (DAFWA)).
Nodulation by the current inoculant strains for beans (WSM1455) and peas (SU303) has been satisfactory down to pH 5.0, noting that the reliability of nodulation at this pH is improved by increased rates of inoculation. Below pH 5.0, even if improved strains become available, the application of lime should be considered as a complementary strategy to improve N-fixation.
Ensure that rhizobia are delivered alive to avoid nodulation failures
Occasional, but sometimes catastrophic nodulation failures have been linked to the mixing of rhizobial inoculants with fertilisers, pesticides or organic amendments. The effects of these practices are often not obvious until a legume is sown oin an inoculation responsive site. Whilst it is recommended that mixing of rhizobia with other amendments is best avoided, it is currently commonplace and so the compatibility of rhizobial inoculants with some commonly used products is being tested.
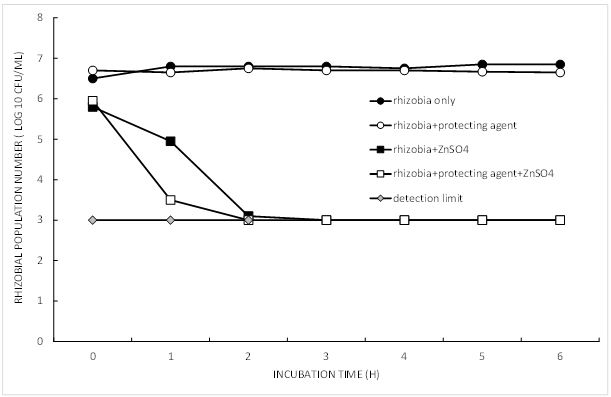
Zinc sulphate provides an example of a product that is used in tank mixes with rhizobia and has been linked to poor nodulation on growers’ fields. Survival of rhizobia was tested in a laboratory simulation of the field situation (Figure 3). Where ZnSO4 was added (125mM, final pH 3.2), populations of rhizobia decreased quickly and were not detectable after 2hrs. Approx. 80% of rhizobia were lost in the first 10mins. (’zero time’ sample). The protecting agent supplied with rhizobia was not beneficial in this instance. When pH and Zn were separated as factors, using tri-K citrate buffer at pH 3.2 and pH 6.8, with and without ZnSO4, the low pH had a much greater inhibitory effect than ZnSO4 on survival of rhizobia (data not shown).
Incompatibility has also been demonstrated in other situations, for example, growers mixing rhizobia with liquid plant nutrient preparations with total salt content EC=96 mS/cm (seawater 55 mS/cm).
Figure 3. Effect of zinc sulphate on the in vitro survival of a freeze dried culture of bean rhizobia.
Some mixtures (for example, ZnSO4 tank mixes with a very low final pH) are to be avoided. In this case, rhizobia are better applied to the seed or added through a second liquid line instead. If mixing rhizobial inoculants with other treatments such as seed pickling fungicides, minimise the exposure time of the rhizobia to the fungicide, apply the inoculant last and sow as soon as possible after coating. Inoculants coated over pesticides also appear to have a better survival with peat than without.
Grow legumes in paddocks with low soil N levels and minimise N fertilisers
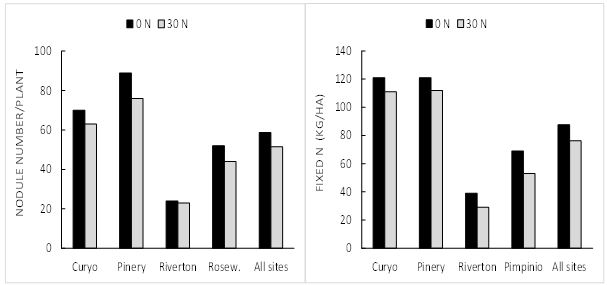
The effect of available N (as soil N or added as fertiliser) is always to reduce N-fixation. The effect of adding 30kg N (urea) at sowing, to mimic increased soil N levels, on field pea nodulation and N-fixation has been examined at five sites (Figure 4). While the reductions with N addition were reasonably small (-12% for nodule number and -13% for N fixed), they were nevertheless highly significant and indicate the potential for reductions in nodulation and N-fixation at higher N supply.
Figure 4. Effect of N fertiliser (30 units of N added at sowing as urea) on the nodulation (left) and amount of N fixed (right) by field peas at five field sites. LSDs for comparing main effect of N addition (all sites mean) is 3.0 for nodulation and 3.8 for N2-fixation.
So, although the effects of small amounts of ‘starter’ N are likely to be minor, as a general principle N-fixation is maximised where available N is minimised.
Be wary of herbicide residues and in crop applications
The contribution of herbicides to the variation in the symbiotic performance of field peas shown in Figure 1 is not known. However, because nodulation was below par at some sites and not able to be improved even with high rates of inoculation, we speculate that herbicides were likely implicated. This is based on evidence of their impact reported in the literature (Anderson et al. 2004, Drew et al. 2007), observations of damage in commercial crops, and positive growth responses provided by herbicide tolerant legumes. It is expected that another project examining the effects of various herbicide groups on strand medic nodulation and N-fixation (Minnipa Research Centre, funded by SAGIT) will provide new information that is relevant to other pasture legumes and pulses.
In the meantime, legume plant back times following herbicide applications should be strictly adhered to, particularly where Group B residues in soil are likely. Where available, herbicide tolerant legume varieties can also be used.
Conclusion
Our work with field peas shows there is scope to improve the amount of N fixed. In principle, this should also be the case for beans, lentils and vetch, which nodulate with the same species of rhizobia. In paddocks where the pulse has previously been grown and there are naturalised populations of rhizobia, increasing fixed N will be achieved by choosing legumes and varieties that have high production and N-fixation potential and managing factors such as soil mineral N and herbicide residues. An on-line tool is being developed that will provide information on the symbiotic potential of different pea varieties. The feasibility of developing a DNA based test to measure the number of rhizobia in soil is also being explored.
At inoculation responsive sites, care needs to be taken with the inoculation process to ensure good nodulation and N2-fixation, and where legume symbioses are likely to be limited by low soil pH, liming should also be considered. If rhizobia with improved acid tolerance continue to perform well with beans, a commercial release is planned within the next three years.
Useful resources
Inoculating Legumes: A Practical Guide
Useful Resources for Legume Growers - The University of Adelaide
GroundCover TV: Episode 20 September 2016
References
Anderson A, et al. 2004. Influence of chlorsulfuron on rhizobial growth, nodule formation, and nitrogen fixation with chickpea. Crop and Pasture Science 55, 1059-1070.
Corbin EJ, Brockwell J and Gault RR, 1977. Nodulation studies on chickpea (Cicer arietinum). Australian Journal of Experimental Agriculture and Animal Husbandry 17, 126-134.
Drew EA, Denton MD, Sadras VO and Ballard RA, 2012. Agronomic and environmental drivers of population size and symbiotic performance of Rhizobium leguminosarum bv. viciae in Mediterranean-type environments. Crop and Pasture Science 63, 467-477.
Drew EA, Gupta VVSR and Roget DK, 2007. Herbicide use, productivity, and nitrogen fixation in field pea (Pisum sativum). Aust. J. Agric. Res. 58, 1204-1214.
Unkovich MJ, Baldock J, Peoples MB, 2010. Prospects and problems of simple linear models for estimating symbiotic N2 fixation by crop and pasture legumes. Plant & Soil 329, 75-89.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, — the author would like to thank them for their continued support.
On mainland SA, trials were managed by the New Variety Agronomy Group (SARDI, Clare and Port Lincoln) and with support from the Hart Field Group. On Kangaroo Island SA, trials were managed by Lyn Dohle and Jenny Stanton. In Victoria, trials were managed by Jason Brand and Frank Henry (Department of Economic Development, Jobs, Transport & Resources), also with support from the Southern Farming Systems Group.
Contact details
Ross Ballard
SARDI Soil Biology and Diagnostics
GPO Box 397, Adelaide, SA, 5001
08 8303 9388
ross.ballard@sa.gov.au
Liz Farquharson
SARDI Soil Biology and Diagnostics
GPO Box 397, Adelaide, SA, 5001
08 8303 9452
liz.Farquharson@sa.gov.au
Maarten Ryder
University of Adelaide, PMB 1, Glen Osmond, SA 5064
0409 696 360
maarten.ryder@adelaide.edu.au
Was this page helpful?
YOUR FEEDBACK