Rhizobia - developing tough strains for tough conditions, inoculant quality and best inoculation practice
Take home message
- A survey of 300 commercial paddocks in central and southern NSW identified multiple widespread soil chemical constraints likely to impact pulse crop yield. Many of these soil constraints were prevalent in the seed-placement zone where there is likely to be impact on the host plant, rhizobia and the capacity for an effective symbiosis to be formed
- Three elite Group E/F strains have been found to have adequate cross-compatibility and capacity to increase nodulation in host pulse crops compared to the current strain in field trials where soil pHCa ranged from 4.6 to 5.0
- Three elite strains have all surpassed growth and manufacturability benchmarks
- Growers should ensure that they always use legume inoculants that display the ‘Green Tick’ logo ensuring they have had independent quality assurance testing
- Minimise the time between inoculant preparation and sowing. Also, avoid mixing inoculants with chemicals (e.g. fertilisers and seed pickles).
Background
The use of high value pulses throughout the GRDC northern region is currently constrained by the adaptation of current commercial rhizobia strains to soil and climatic conditions. Of these constraints: soil acidity, soil texture (low clay content), aridity and genetic stability of rhizobial strains are key factors limiting pulse production via impacts on rhizobial survival and effectiveness.
Where pulses do not adequately nodulate or a less than optimal symbiosis is formed with less effective rhizobia, nitrogen fixation, crop growth, grain yield and nitrogen (N) carryover to subsequent crops can be compromised. Such limitations are constraining the use of pulses in cropping rotations. The objectives of this project, which links to similar projects in the GRDC western and southern regions, is to quantify the impact of a range of elite rhizobial strains on grain yield of pulses and N supply and benefit of pulses to future crops in the rotation. The project is also investigating strategies that minimise possible adverse impacts of management practices on nodulation and N fixation. Such practices include, time of sowing, soil moisture at sowing, desiccation of rhizobia on seed and herbicide impacts. Ultimately the aim is to deliver to industry, rhizobial strains and appropriate management to increase grain legume adaptation, production and carryover benefits to following crops in the rotation. This project is currently underway, and this paper will present some results to date as well as a snapshot of what is planned.
Soil and management constraints that impact nodulation and N-fixation: A survey
The project team have collaborated extensively with Pulse Check and farming system groups throughout the project. A large extension program was undertaken in 2019 to identify soil and management constraints throughout the GRDC northern region. A total of 300 paddocks were tested across 150 mixed farming properties. For each paddock, composite soil samples were collected for the depth 0-5 cm, 6-10cm, 11-20cm and 21-30 cm layers. Each grower completed a survey documenting 5-year management history, for the sampled paddock, as well information on the type, magnitude and frequency of other constraints to production at the farm scale. The purpose of the survey was to identify regional and sub-regional issues impacting pulses (and other crops and pastures in the rotation) in order to develop an extension program to improve pulses which integrated yield and profitability and integrated with elite rhizobial strain development. Our survey revealed widespread soil constraints including soil acidity (particularly prevalent in the south east) and nutrient deficiency specifically phosphorus (P) and sulphur (S) availability (Hackney et al. 2020). Soil acidity and poor soil nutrition can impact the survival and growth of rhizobia as free-living saprophytes (that is, their survival in the soil in the absence of the host plant) as well as during their symbiotic relationship with legumes (O’Hara, 2001).
Tough rhizobia for tough conditions: selecting elite rhizobial inoculants to improve outcomes in hostile soil conditions
For successful nodulation and N2 fixation to occur both the host plant and its rhizobial partners must be suited to the soil and environmental conditions into which they are planted. Rhizobia must be delivered on or with seed in sufficient numbers to compete with background rhizobial strains to adequately nodulate a host plant and fix N2. Legumes that form a sub-optimal symbiosis may fix insufficient N2 and will deplete soil mineral N rather than building it.
New elite rhizobia for faba/ lentil/ pea and vetch growing in acid soils
Currently the commercial rhizobial recommendation for Group E legume hosts including field pea and vetch is SU303. The recommended commercial strains for Group F legume hosts including faba bean and lentil is WSM1455. In reality, due to the difficulty in manufacturing SU303, the strain WSM1455 which has a broad host range is used for both Group E and F legume hosts. The current Group F which was isolated from soil in Greece (pHCa 8.0) and released in 2002, exhibits a rapid decline in capacity to nodulate plants where pHCa < 6.0, with plants generally inadequately nodulated where pHCa <5.0 (Yates et al. 2016; Ballard et al. 2019) therefore a new acid-tolerant replacement strain is being sought. Ideally, a replacement strain for this group would cover the entire host range, that is nodulate and fix N on faba, lentil, pea and vetch.
A range of new ‘acid-tolerant’ rhizobial strains (SRDI969, SRDI970, WSM1483 and WSM4643) have been developed and shortlisted by South Australian Research and Development Institute (SARDI) and the Centre for Rhizobium Studies (CRS), Murdoch University, Western Australia. Both groups isolated these elite strains from acid soils (pHca 4.5-5.5) from cropping paddocks in South Australia (SRDI strains) and Italy (WSM strains) respectively. Preliminary glasshouse and field studies in Western Australia identified two acid-tolerant Italian strains (WSM1483, WSM4643) which produced improved nodulation, N-fixation and grain yield in field pea (GRDC, 2017). Similar studies in South Australia reported improved performance in lentil and faba bean where acid tolerant Australian strains (SRDI969, SRDI970) were used compared to the current Group F (WSM1455) strain (Ballard et al. 2019).
Before a recommended commercial rhizobial strain for a major inoculant host group is changed, robust scientific evidence must be gathered that the new elite strains are superior to current strains in all aspects including: manufacturability (scalability and shelf-life), genetic stability (reduced tendency to loose genes for nodulation), desiccation tolerance, legume host range and performance in the field (nitrogen fixation, grain yield). It is imperative that for a strain change of such importance, that several field trials are conducted over multiple seasons in different soil conditions and types and on a broad host range. This paper presents the results of trials in NSW in 2019. Field trials have also been conducted in both South Australia and Western Australia in 2019 and are currently underway for 2020. The tolerance of these strains to adverse management impacts such as herbicides and their residues will also be assessed in this project.
Manufacturability, genetic stability and desiccation tolerance
The purpose of this component of the project is to determine if the new elite acid-tolerant strains can be manufactured in a peat carrier and reach sufficiently high numbers (i.e. 1 X 109 CFU/g) to meet the standards specified in the National Code of Practice for Legume Inoculants. The current Group E (SU303), current Group F (WSM1455), or new elite acid tolerant strains SRDI970, SRDI969, WSM1483 and WSM464 were used to produce peat inoculant and measured to determine number of rhizobia. All strains met or exceeded the minimum standard for peat inoculants in the National Code of Practice for Legume Inoculants, that is greater than 1 x 109 CFU/ g peat. All strains held this high number of rhizobia when stored for 6 months at 4 °C. However, there was a drop in rhizobial numbers if stored under non-optimal conditions at room temperature. The long-term viability of these strains is still being assessed (an experiment is underway) with the goal of having appropriate expiry date recommendations for products at the time when the new commercial strain is recommended. Experiments are currently underway to assess the survival of these strains on seed. In particular, an industry minimum standard will be quantified for each strain (that is, 104 CFU for medium seed like lentil and 105 CFU per large seed like faba, lupin, pea). Assessments of genetic stability are ongoing, however, the full genomes of three of the elite strains has been sequenced. This will enable better understanding of genetic characteristics of the different strains.
Performance in the field
In 2019, field testing of the four elite acid-tolerant strains commenced in the Northern GRDC region with sites sown at Griffith, Condobolin and Canowindra. The objective of the three sites was to evaluate the performance of the elite strains in differing soils (Table 1) and under varying climatic conditions. Griffith was the most challenging of the sites due to lower soil pH, low clay content and high summer temperatures. Canowindra with a higher soil pH, higher clay content and lower temperatures potentially increased survival capacity of the rhizobia, although such conditions also increase the potential for higher background rhizobia levels that compete with introduced strains.
Table 1. The location, soil pH and texture of three sites used for evaluation of elite rhizobia strains for lentil, faba bean, field pea and vetch in the GRDC Northern Region in 2019.
Location | Soil pHCa (0-10 cm) | Soil texture |
|---|---|---|
Griffith | 4.6 | Light sandy loam |
Condobolin | 4.8 | Sandy loam (red chromosol) |
Canowindra | 5.1 | Loam (dermosol) |
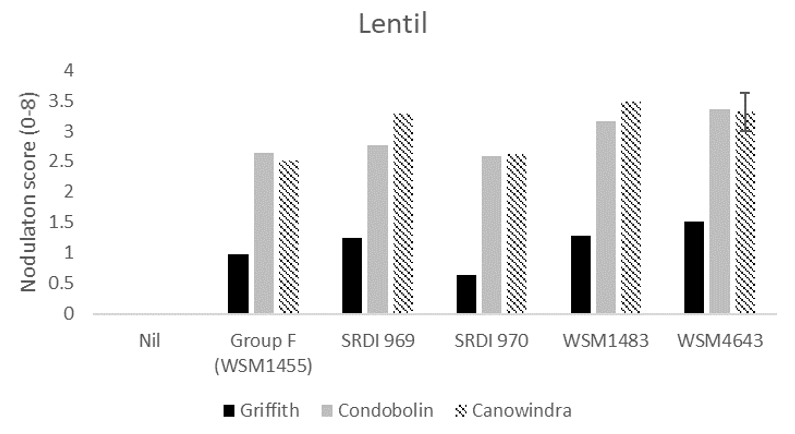
For lentils, there was a significant difference in nodulation across sites and due to rhizobia strain (Figure 1). The most acidic site, Griffith, had lower nodulation than all other sites and only WSM4643 produced a significantly higher nodulation score than the current Group F strain. At Condobolin and Canowindra, both WSM1483 and WSM4643 produced significantly higher nodulation scores than the current Group F, while SRDI969 was also significantly higher than the current Group F at Canowindra. None of the strains at any site gave an overall nodulation score that is considered adequate, adequate (score ≥ 4) is described as 21–40 small pink and/or 3–4 large pink nodules (Yates et al. 2016).
Figure 1. The average nodulation score of 15 lentil plants at Griffith, Condobolin and Canowindra where seed was inoculated with peat slurry containing a no rhizobia (nil), the current Group F strain, or one of four experimental strains. A score of 4 is considered adequate which is described as > 20 effective per plant Yates et al. (2016). The interaction Least Significant Difference (LSD) is indicated.
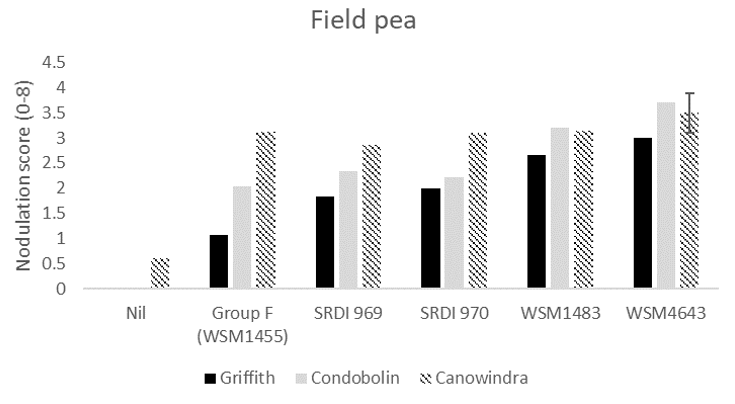
For field pea, all experimental strains produced significantly greater nodulation than the current Group F at Griffith (Figure 2). At Condobolin and Canowindra, WSM4643 produced significantly higher nodulation score than the current Group F, with WSM1483 also significantly higher at Condobolin.
Figure 2. The average nodulation score of 15 field pea plants at Griffith, Condobolin and Canowindra where seed was inoculated with peat slurry containing a no rhizobia (nil), the current Group F strain, or one of four experimental strains. A score of 4 is considered adequate which is described as > 20 effective per plant Yates et al. (2016). The interaction Least Significant Difference (LSD) is indicated.
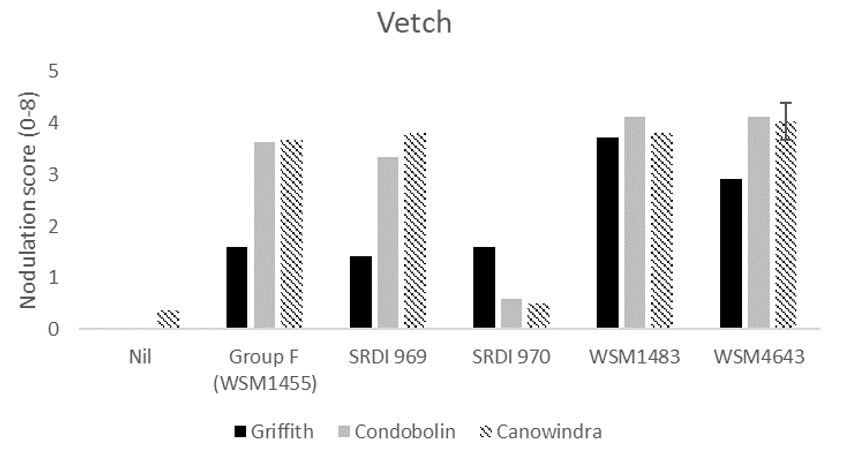
For vetch, WSM1483 and WSM4643 produced significantly higher nodulation than the current Group F at Griffith and Condobolin (Figure 3). None of the experimental strains produced significantly higher nodulation than the current Group F at Canowindra. SRDI970 showed poor compatibility with vetch at Condobolin and Canowindra producing very low nodulation scores. It is critical that strains considered for potential release showed strong compatibility across the potential host range.
Figure 3. The average nodulation score of 15 vetch plants at Griffith, Condobolin and Canowindra where seed was inoculated with peat slurry containing a no rhizobia (nil), the current Group F strain, or one of four experimental strains. A score of 4 is considered adequate which is described as > 20 effective per plant Yates et al. (2016). The interaction Least Significant Difference (LSD) is indicated.
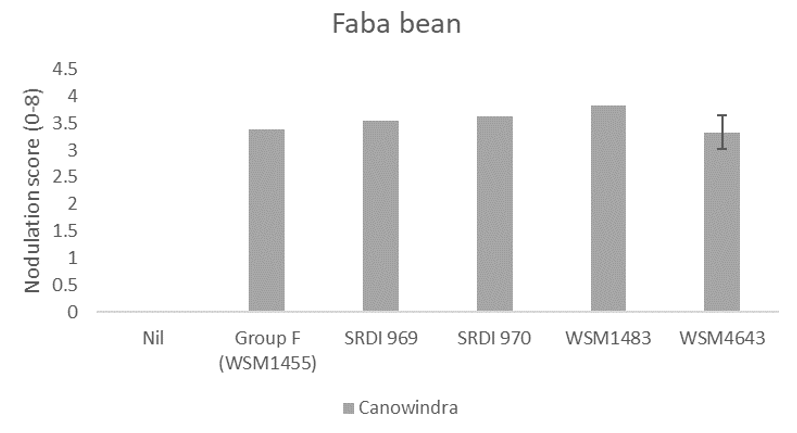
Faba beans were grown only at Canowindra and there were no experimental strains that gave nodulation scores greater than those achieved by the current Group F strain (Figure 4).
Are tough rhizobia enough for tough conditions?
Developing new elite strains that have improved tolerance to soil acidity is certainly a step in the right direction. However, it’s important to consider that without addressing the issue of soil acidity this condition will only continue to worsen. While the elite WSM strains increased nodulation compared to the current Group F for most species at most sites, nodulation was still not within the range considered adequate for the legumes grown (score ≥4, equivalent to >20 effective nodules, Yates et al. 2016). This may indicate that nodulation was supressed by other factors (such as drought or acidity). Acidity was observed to be impacting the host plant directly in some cases, especially for lentil at the most acidic site. This indicates that even with acid tolerant rhizobia there is likely to still be constraints to production.
Herbicide impacts
Herbicides may impact biological N-fixation by affecting the rhizobia directly, the legume host or both simultaneously and therefore their capacity to form an effective symbiosis. Common post-sowing and pre-emergent herbicides used in legumes or residues of herbicides used in previous crops have the potential to cause reductions in plant density, crop yellowing, distortion of plants (above and below ground) and reductions in nodulation, N-fixation, yield and N carryover to subsequent crops. Whilst this work is ongoing, this project has started to consider the direct and indirect impact of herbicides and their residues on rhizobia, especially for the current commercial strains (WSM1455 & SU303) and new elite rhizobia (SRDI969, SRDI970, WSM1483, WSM4683) under consideration for legume host groups E/F (Faba, Lentil, Pea and Vetch). We performed an in vitro laboratory assay assessing the direct toxicity of various herbicides to rhizobia. It should be noted that such an in vitro study does not reflect the complex situation that exists in a soil system and does not consider the complex issues of herbicide bioavailability and the symbiotic relationship between the plant host and rhizobia. We found that the herbicides that contained active ingredients glyphosate, 2,4 D amine, metsulfuron methyl and a combination of dicamba and MCPA (4-chloro-2-methyphenxy acetic acid) were most toxic directly at high concentrations (closer to when herbicides are applied than residual concentrations in soil) to the rhizobial strains tested. Our next study (planned for late 2020) will measure the impacts of a range of herbicides and their residues on both legume hosts and their rhizobial partners and quantify the impact of these chemicals on nodulation and N-fixation. There are currently two field trials in at the moment evaluating the effect of herbicide residues on legume growth, nodulation and nitrogen fixation. So far, residues of dicamba+MCPA, Group B herbicides (e.g. metsulfuron methyl), clopyralid are proving particularly damaging across all host legumes causing yellowing, distortion and in some cases, significant reductions in stand density.
Assurance of high-quality rhizobia inoculant products: independent ‘Green Tick’ quality assurance program
Ensuring that new and compatible rhizobia are available to successfully nodulate Australia’s evolving selection of grains and pastures is critical. The Australian Inoculant Research Group (AIRG) is playing an important role in rhizobial strain selection, testing, evaluation and recommendation for pulses ensuring that suitable rhizobia will be effective and persistent in Australia’s challenging growing conditions. This level of independent scrutiny provides a high degree of confidence to growers that Australian rhizobial inoculant products on store shelves are precisely ‘fit for purpose’.
The Australian Inoculants Research Group (AIRG), ensures that Australians commercial inoculant manufacturers have access to standardised and authenticated commercial rhizobial strains for production of their products. Each year before the commercial strains are issued to industry we ensure that they are what they are supposed to be (correct strain), they can still form effective nodules with the host legume (in other words they haven't lost their ability to nodulate a host) and that genetic drift of the commercial strains is minimised.
Figure 5. The Green Tick on rhizobial products signifies that the AIRG have tested and are shown to be ’fit for purpose’.
Growers can be confident that a product purchased in store endorsed with a Green Tick is optimised for success because it has been quality assured via the rigorous, independent testing process of the AIRG. Products that carry the quality assurance Green Tick logo contain the correct strain, and minimum number of viable rhizobia and are free from contaminants as labelled at the point of manufacture.
Inoculants that display the Green Tick logo have met the requirements of the National Code of Practice and Quality Trademark for Legume Microbial Inoculant Products. These products have been independently assessed by us to ensure that inoculants (at the point of manufacture) have: the approved, correct and viable strain of rhizobia for the legume to be planted; guaranteed numbers of live rhizobia required to successfully nodulate the legume to be planted- this is 1 billion/gram for fresh peat products; nil or minimal numbers of other non-rhizobia micro-organisms that may impact upon the performance of the rhizobia; optimal moisture content for growth and survival of the rhizobia along the supply-chain until sowing and can still nodulate the intended host legume post manufacture at the indicated expiry date.
Only when these standards have been met is the batch of inoculant approved for sale, with an expiry date. While we can guarantee rhizobia numbers at the time of testing, it is critical to check with your retailer that the product has been transported and stored appropriately to ensure viability at the point of sale. Once purchased, you need to observe storage requirements to ensure viability at the point of sowing.
Green Tick inoculants have labelling standards that require manufacturers to display the Green Tick logo and clearly indicate: the legume/s for which the inoculant should be used; batch number; product shelf-life or expiry date; conditions under which the product must be stored; guaranteed number of rhizobia per unit of product at the point of sale; and method of application to seed or soil of the product.
Transparent labelling, underpinned by independent testing, provides a solid foundation for nodulation success and, therefore, profitable production outcomes.
Key considerations to ensure the best inoculation outcomes
Growers should consider the following to ensure successful nodulation and improve N-fixation:
- Selection of an appropriate inoculant group for each legume
- Check that inoculant products have been stored appropriately in store before purchase. After purchase store inoculants correctly; avoid hot conditions and direct sunlight. Follow the storage directions on each inoculant pack
- Time of sowing; - minimise the time between preparation of inoculant products and sowing. Rhizobia are living organisms and are susceptible to desiccation, extremes of temperature and exposure to chemicals which can be toxic. The number of rhizobia will begin to decline once a product is mixed, therefore inoculants should be prepared only immediately prior to their intended use and sowing for seed. One factor in successful nodulation relies on rhizobial numbers, the more rhizobia present the greater the chance of improving nodulation and N-fixation (the minimum number of cells is 103, 104, 105 for small, medium and large seeded legumes). Soil moisture levels at the time of sowing have a significant impact on rhizobial survival, moist conditions are best
- Minimise the time that rhizobial strains are exposed to seed applied pesticides (e.g. seed pickles). If possible, select seed treatments with low impact on rhizobia and sow seed as soon as possible after treating with rhizobia
- Consider herbicide label legume plant back recommendations. Be mindful of both the time since last use as well as soil moisture requirements for plant-back periods
- When using tank mixed or liquid inoculants do not add anything that is toxic to rhizobia e.g. some fertilisers, trace elements, fungicides, insecticides etc.
References
Ballard R, Farquharson E, Ryder M, Denton M, Rathjen J, Henry F, Brand J, Whitworth R, Haskins B, Seymour M, Yates R (2019). Fixing more N – improving the performance of rhizobial inoculants in suboptimal conditions. GRDC Updates.
Drew, E., Herridge, D., Ballard, R., O’Hara, G., Deaker, R., Denton, M., Yates, R., Gemell, G., Hartley, E., Phillips, L., Hara, G.O., Deaker, R., Denton, M., Yates, R., Gemell, G., Hartley, E., Phillips, L., Seymour, N., Howieson, J., Ballard, N., (2012). Inoculating Legumes: A practical guide, Grains Research and Development Corporation.
GRDC (2017) Groundcover article Accessed 15 July 2020
Hackney, B., Rigg, J., Flinn, S., Menz, I., Gill, W. Thompson, C., Bartimote, T., Edwards, C. and Orgill S (2020) Soil and management impacts on potential legume production: results of a survey of 300 commercial paddocks in the GRDC northern region. GRDC Grains Research and Development Update, 13 August 2020
O’Hara, G.W., (2001). Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: A review. Animal Production Science 41(3):417-433
Yates, RJ, Abadidoo, Howieson, JG (2016) Field experiments with rhizobia. In. Working with Rhizobia (Eds. JG Howieson, MD Dilworth). Australian Centre for International Agricultural Research and Centre for Rhizobium Studies Murdoch University.
Acknowledgements
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the author would like to thank them for their continued support. The authors also wish to acknowledge the financial and in-kind contributions of Local Land Services and Central West Farming Systems towards the soil survey results presented in this paper.
Contact details
Jessica Rigg
Soil & Water R & D, NSW DPI
Elizabeth Macarthur Agricultural Institute
Woodbridge Rd, Menangle
Email: jessica.rigg@dpi.nsw.gov.au
GRDC Project Code: DAN1901 002 RTX,
Was this page helpful?
YOUR FEEDBACK