Azole resistance in spot-form net blotch in Western Australia
Author: Fran Lopez-Ruiz, Wesley Mair, Geoff Thomas, Kith Jayasena, Andrea Hills, Anke Martin | Date: 11 May 2020
Key Messages
- Fungicide resistance to Group 3 (DMI) fungicides in spot-form net blotch is spreading in the southern region of Western Australia.
- Overuse of fungicides with the same mode of action will speed up the development of fungicide resistance.
- The development of fungicide resistance can be limited by using the lowest effective label dose, appropriate fungicide group rotations and employing integrated disease management (IDM) practices including crop rotation, stubble management and selection of more resistant cultivars (if available).
Introduction
Foliar fungal diseases are an important limiting factor to the yield and quality of broadacre crops worldwide. Control of fungal diseases in broadacre crops relies heavily on the use of broad spectrum single-site fungicides. Unfortunately, continuous application of fungicides has led to the selection and development of fungicide resistant populations in many fungal pathogens, reducing the management alternatives available for growers and threatening profitability worldwide.
In Australia, several major grain-crop diseases have recently developed resistance to some fungicide actives sparking concerns within the industry. The limited availability of cheap and effective chemistry, together with the use of tight rotations in many cases, have contributed to the fast onset of resistance. Since 2012, ten cases of fungicide resistance and four cases of reduced sensitivity (resistance that has not reached the level of field failure) have been reported in Australia (Figure 1).
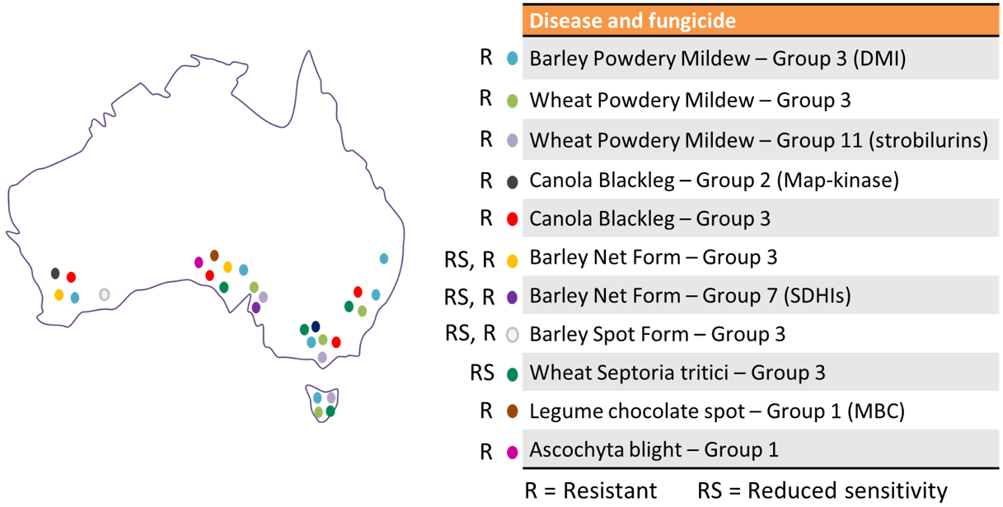
Figure 1. Map of Australia showing presence of Resistant or Reduced Sensitive (resistance that has not reached the level of field failure) fungal pathogens in regions of Australia to specific fungicides within different modes of action. Coloured points indicate general locations.
Barley spot-form net blotch (SFNB) is caused by Pyrenophora teres f. maculata (Ptm) and is the most significant necrotrophic disease of barley in Australia (Murray and Brennan, 2010). Along with cultural practices, the main control measures are the application of effective fungicides and the use of cultivars with genetic resistance. However, due to the lack of highly resistant cultivars, fungicides are a key component of control programs for SFNB (Sierotzki et al 2007).
In recent years, the discovery of hybrid isolates between Ptm and the causal agent of barley net-form net blotch (NFNB), Pyrenophora teres f. teres (Ptt) (Poudel et al 2017), has opened up the door to the possibility of hybrid populations with increased ability to adapt to crop conditions being selected in barley paddocks. In 2017, the Department of Primary Industries and Regional Development (DPIRD) reported a barley paddock near South Stirling, Western Australia in which, despite the application of a strong IDM program, SFNB was found at extremely high levels (Figure 2).

Figure 2. A field trial in Western Australia (WA) showing symptoms of SFNB infection, following treatment with: Tebuconazole, 400mL/100kg (SD); Propiconazole, 325mL/ha @ Z25; Cyproconazole + Azoxystrobin, 400mL/ha @ Z31; Epoxiconazole, 250mL/ha @ Z39 and Propiconazole, 500mL/ha @ Z52.
The efficacy of some Group 3 fungicides has been impacted by the development in WA of a resistant hybrid that shares many similarities with SFNB. This paper provides an overview of the current knowledge of this fungicide-resistant hybrid and the spread of the resistant populations in WA.
Method
Fungal isolates and in-vitro fungicide resistance analysis
Two hundred and sixty-eight Ptm fungal isolates collected from WA barley-growing regions between 1996-2019 were used in this study (for isolation conditions please see the procedure described by Mair et al 2016). Isolates were tested on a set of fungicide discriminatory concentrations established through previous analyses (Mair et al 2016; Lopez-Ruiz et al 2019) to determine if resistance to the fungicides tebuconazole, epoxiconazole (Group 3, DMI), boscalid (Group 7, SDHI) and azoxystrobin (Group 11, strobilurins) was present.
A subset of 23 isolates able to grow above discriminatory concentrations were subjected to further in vitro fungicide sensitivity analysis by growing the cultures at different concentration ranges of seven DMI fungicides (Figure 3). This analysis allowed determination of the fungicide concentration that inhibits the growth of each isolate by 50 per cent, also known as the effective concentration 50 (EC50). Resistance factors (RF) were determined as the fold-number difference between EC50 values from resistant isolates and the average of 22 sensitive isolates known as the wild type population in this study.
Molecular analysis of fungicide resistance
Isolates able to grow above discriminatory concentrations were subjected to polymerase chain reaction (PCR) and sequencing analysis for the detection of the known DMI fungicide resistance mutation F489L (Lopez-Ruiz et al 2019) and other mutations that could be associated with the resistance levels detected. The wild type population was used as a reference in this study.
Hybridisation analysis and population studies
Six specific genetic markers for each form of P. teres (Ptt and Ptm) that enable unambiguous identification of hybrids (Poudel et al 2017) were used to analyse resistant isolates for potential hybridisation. DNA from all isolates was extracted as described by Mair et al (2016) and submitted to sequence-specific PCR with the aforementioned markers.
A Diversity Arrays Technology (DArT) marker analysis was used to determine the level of genetic diversity within a subsample of 40 Ptm resistant isolates collected from WA. Seven sensitive and reduced sensitive isolates, three Ptt isolates and two barley grass P. teres isolates were included as a reference. DNA from all isolates was extracted as above and a total of 9656 silicoDArT markers were used. Similarity matrix was constructed with DICE coefficient and cluster analysis of matrix values by UPGMA.
Results
High levels of fungicide resistance to some Group 3 fungicides in SFNB
The fungicide sensitivity analysis of the 268 Ptm isolates collected between 2014 and 2019 from barley fields revealed three different groups based on their levels of sensitivity to the DMI fungicide tebuconazole: ‘sensitive’, ‘reduced sensitive’ (resistance that did not reach the level of field failure) and ‘resistant’. Interestingly, resistant isolates (19.4%) were only found from the 2016 season onwards and were limited to the southern region of WA (Figures 3A and B). In 2018, a highly sensitive molecular detection methodology was developed for the analysis of mutations associated with the different levels of fungicide sensitivity in Ptm (Lopez-Ruiz et al 2019). The use of this technique increased the levels of ‘reduced sensitivity’ detections quite dramatically (Figures 3A and B). Further work is required to determine the levels of ‘reduced sensitive’ populations between 1996-2018 with this new technique.
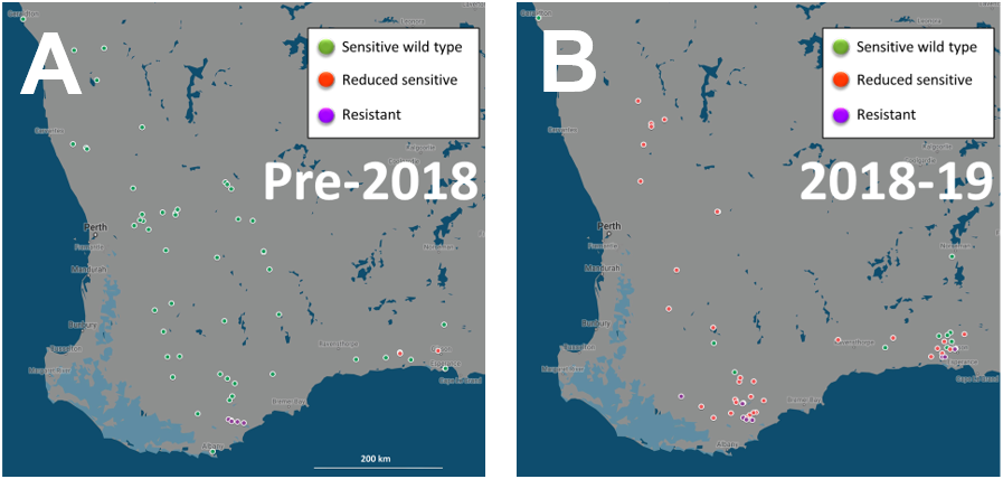
Figure 3. Maps of Western Australia showing geographic origin of spot form of net blotch (SFNB) samples collected in 1996-2018 (A) and 2018-2019 (B) and tested for resistance to the Group 3 fungicide tebuconazole using fungicide discriminatory tests (A) and mutation detection techniques (Lopez-Ruiz et al 2019) (B). Coloured dots indicate presence of sensitive (green), reduced sensitive (red) and resistant (purple) isolates. In many cases multiple samples were obtained from the same location. Samples from the 2019 season are still being analysed so results are just partial at this stage.
A subset of 23 putatively-resistant isolates were further analysed using a microtiter test for sensitivity to seven DMI compounds as outlined by Mair et al (2016) (Figure 4). EC50 values to the seven DMI compounds were used to sort the strains into four different phenotypic groups. Two ‘reduced sensitive’ categories (reduced sensitive 1 and 2) were defined based on the phenotypic differences observed (red and orange lines, respectively in Figure 4). However, these two ‘reduced sensitive’ groups are not different from a field management point of view so are represented together in the distribution maps (Figure 3).
Compared to the ‘sensitive’ reference, reduced sensitive-1 isolates had higher EC50 values for all DMI compounds tested. Interestingly, reduced sensitive-2 isolates had EC50 values higher than those of the ‘sensitive’ reference for all compounds tested except for epoxiconazole (mean 0.09µg/mL). The resistant isolates had EC50 values higher than the sensitive and reduced sensitive-1 and -2 isolates for the compounds difenoconazole (mean 1.67µg/mL), metconazole (mean 11.0µg/mL), prochloraz (mean 2.81µg/mL), propiconazole (mean 6.76µg/mL), prothioconazole (mean 1.77µg/mL), and tebuconazole (mean 17.1µg/mL), higher EC50 values than that of sensitive and reduced sensitive-2 isolates and similar EC50 values to reduced sensitive-1, for epoxiconazole (mean 1.62µg/mL).
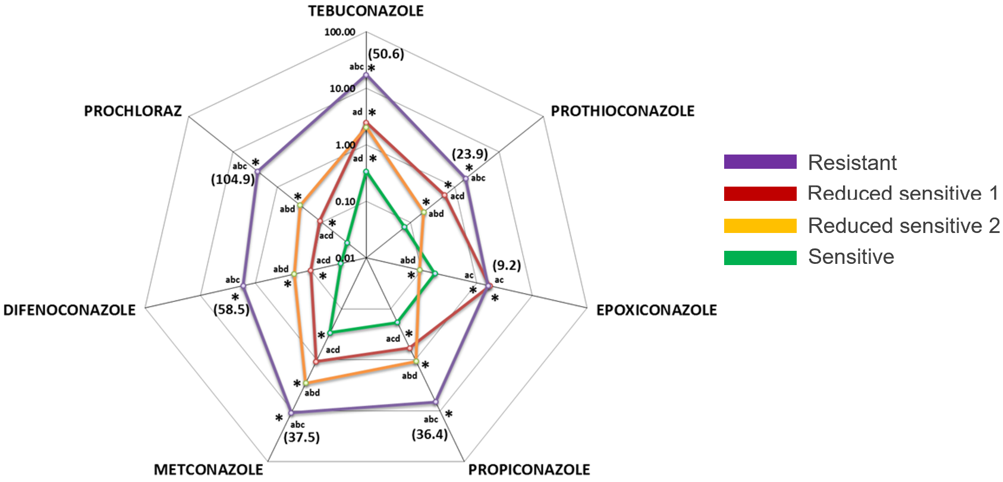
Figure 4. Average EC50 (µg/ml) of Ptm sensitivity groups to seven DMI fungicides. Four different fungicide sensitivity groups are shown: sensitive (green), reduced sensitive-1 (red), reduced sensitive-2 (orange) and resistant (purple). Values in brackets represent resistant factors. *The mean difference between groups aS, bMR1, cMR2 & dHR is significant at 0.05 level (Kruskal-Wallis H test & Dunnett’s T3)
Fungicide actives, tebuconazole and propiconazole were among the Group 3 compounds most compromised by the mutations found in the resistant isolates. The efficacies of epoxiconazole and prothioconazole were less affected. High RF and EC50 values, or growth of the isolates at discriminatory doses does not necessarily imply field failure as this will depend on the frequency of the resistance in the paddock – further studies are needed to determine this.
Mutations in the Group 3 target gene are correlated with fungicide resistance in SFNB
Two main gene changes were found to be correlated with resistance to Group 3 fungicides in SFNB. The genetic analysis of resistant isolates revealed the presence of mutation F489L in all resistant isolates. This mutation had been previously described in NFNB by Mair et al (2016). In addition to the F489L mutation, resistant isolates also carried a major modification in a region of the gene that controls the levels of the target in the fungus. This modification allowed resistant isolates to produce higher levels of the Group 3 target resulting in lower sensitivity to all DMI fungicides tested (Figure 4).
Hybridisation and population analysis reveal that resistant isolates are clonal hybrids between Ptt and Ptm
The hybridisation analysis of the isolates revealed that all fungicide-resistant isolates found in this study were positive for all Ptm species-specific markers. In addition, all resistant isolates were also positive for 1/6 Ptt species-specific markers.
Population marker analysis showed that all resistant isolates used in the study clustered together but away from Ptt or P. teres isolates collected from barley grass. Resistant isolates did not cluster with Ptm isolates but results indicated a close relationship between them, and this agreed with the hybridisation results (Figure 5).
The analysis also revealed a stunning lack of genetic diversity among the 40 resistant isolates tested. The majority of resistant isolates tested were in fact genetically identical (or clonal), even though the clones were collected from seven different locations in the South Stirling and Esperance regions and separated by distances of up to 350km. The results suggest that these isolates are part of a clonal population that has spread across the barley growing regions in the south of WA.
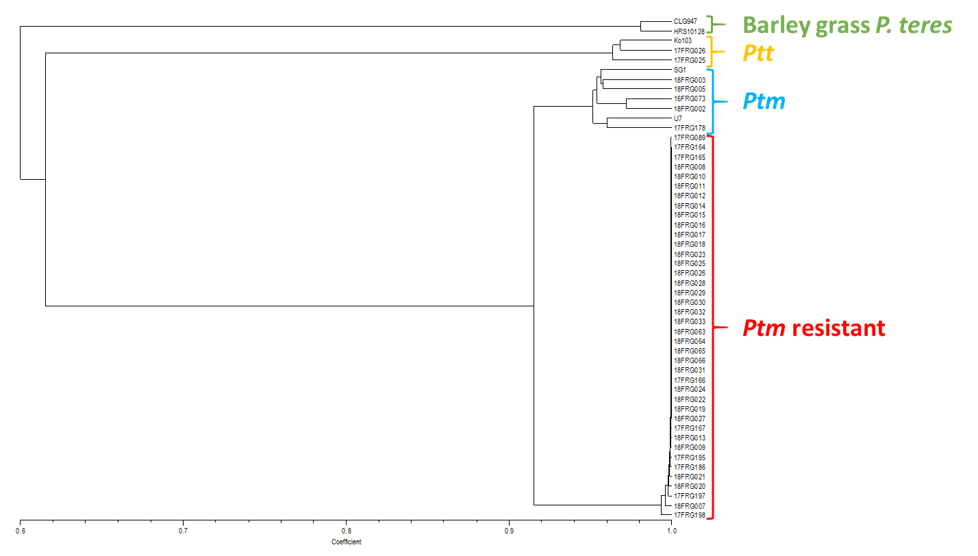
Figure 5. Phylogenetic tree based on DArT marker analysis showing the genetic relationships between 40 Ptm resistant isolates and two barley grass P. teres isolates, three Ptt isolates and seven Ptm isolates.
Conclusion
Fungicide resistance has developed in SFNB in WA. Analysis of collected isolates showed that all resistant isolates belonged to a hybrid clonal population that has spread across the south of WA. Interestingly, resistant isolates (19.4%) were only found from the 2017 season onwards, which suggests a recent development in resistance to DMIs in the region.
Most of the resistant hybrid fungicide isolates collected so far have come from infected Oxford samples. Variety tests carried out by DPIRD showed that the hybrid was slightly more virulent on Oxford than comparatively more sensitive reference isolates. In addition, the hybrid isolates seemed to be less competitive on other varieties. This probably indicates an increased level of genetic compatibility between Oxford and the hybrid resistant isolates. It is important to know that the only two other varieties where these hybrid fungicide-resistant isolates have been found are Planet (PBR) and La Trobe (PBR), albeit at far lower frequencies and in regions with a history of Oxford production. This information suggests that improved knowledge of host susceptibility could enable selection of varieties with lower susceptibility to this hybrid isolate. This could lead to a reduction in the population of the hybrid and slow its spread.
Fungicide actives, tebuconazole and propiconazole were among the Group 3 compounds most compromised by the mutations found in the resistant isolates. Therefore, in the high rainfall areas of Esperance and Albany where the resistant type has been detected, their use should be avoided in solo formulations and limited when any of these two compounds are mixed with a different fungicide, especially from Group 3, as this will place extra pressure on the other mixing partner. In areas where only the ‘reduced sensitive’ type has been detected (central and northern regions), propiconazole use should be limited to once per season to avoid placing extra pressure on this active.
Other fungicide modes of action, such as those from Group 11 (QoIs) and Group 7 (SDHIs) remain effective at controlling these hybrids and should be incorporated into spray programs. However, the use of SDHI fungicides should be monitored carefully given the recent discovery of SDHI resistance in NFNB in South Australia.
Acknowledgments
The research undertaken as part of this project is made possible by the significant contributions of growers through both trial cooperation and the support of the GRDC, the authors would like to thank them for their continued support.
The authors would also like to acknowledge the contribution of The Foundation for Arable Research in Australia (FAR) and the grains industry.
Special thanks go to growers and grower groups who submitted samples to make this research possible.
References
Murray, G.M. and Brennan, J.P. (2010). Estimating disease losses to the Australian barley industry. Aust. Plant Pathol. 39,85–96.doi:10.1071/AP09064.
Sierotzki, H., Frey, R., Wullschleger, J., Palermo, S., Karlin, S., Godwin, J., et al. (2007). Cytochrome b gene sequence and structure of Pyrenophora teres and P. tritici-repentis and implications for QoI resistance. Pest Manag. Sci. 63, 225–233.doi:10.1002/ps.1330.
Mair, W.J., Deng, W., Mullins, J.G.L., West, S., Wang, P., Besharat, N., Ellwood, S.R., Oliver, R.P. and Lopez-Ruiz, F.J. (2016). Demethylase Inhibitor Fungicide Resistance in Pyrenophora teres f. sp. teres Associated with Target Site Modification and Inducible Overexpression of Cyp51. Front. Microbiol. 7:1279. doi: 10.3389/fmicb.2016.01279.
Lopez-Ruiz, F., Mair, W., Thomas, G., Jayasena, Kith and Hills, A (2019). The incidence of fungicide resistance in spot form net blotch (SFNB) and its implications. https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2019/02/the-incidence-of-fungicide-resistance-in-spot-form-net-blotch-sfnb-and-its-implications.
Poudel, B., Ellwood, S.R., Testa, A.C., McLean, M., Sutherland, M.W. and Martin, A. (2017). Rare Pyrenophora teres Hybridization Events Revealed by Development of Sequence-Specific PCR Markers. Phytopathology, 107(7): 878-884.
Contact details
Fran Lopez-Ruiz
Centre for Crop and Disease Management, Curtin University
Kent Street, Building 303, Bentley, WA 6102
08 9266 3061
fran.lopezruiz@curtin.edu.au
GRDC Project Code: CUR00023,
Was this page helpful?
YOUR FEEDBACK
